Abstract
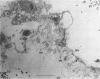
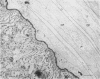
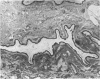
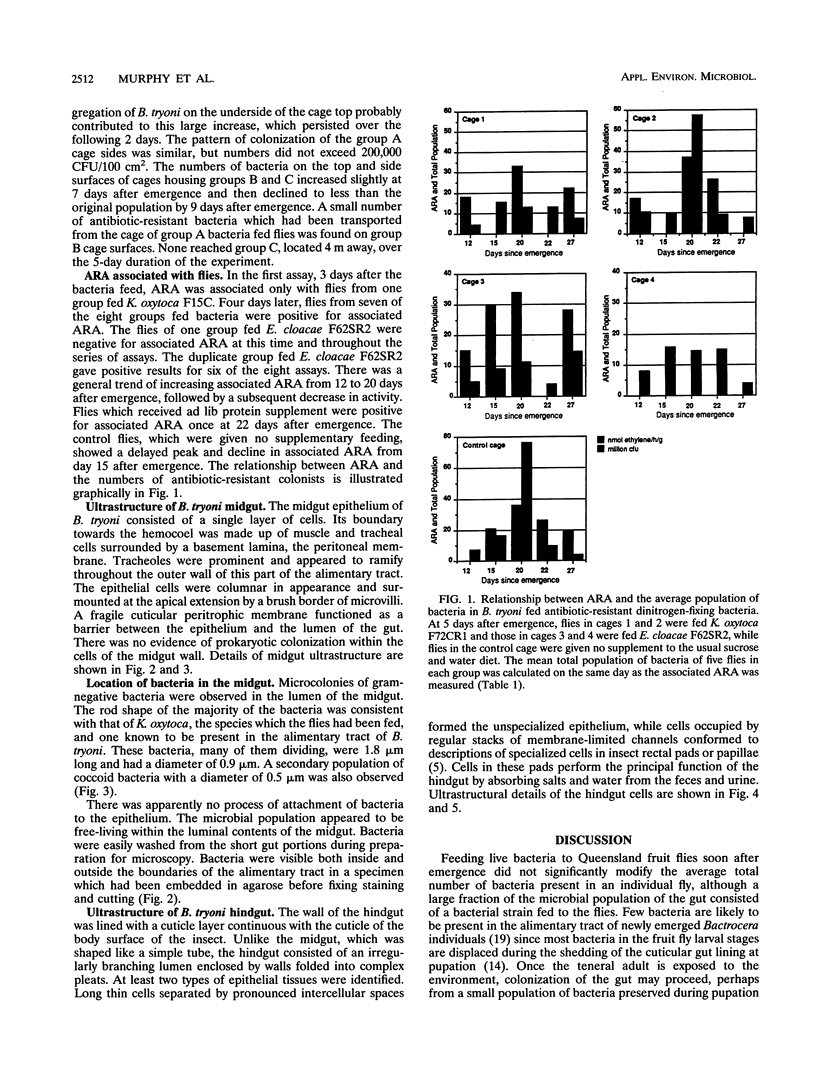
The average total population of bacteria remained constant in the alimentary tracts of adult laboratory-raised Queensland fruit flies (Bactrocera tryoni) although the insects had ingested large numbers of live bacteria as part of their diet. The mean number of bacteria (about 13 million) present in the gut of the insects from 12 to 55 days after emergence was not significantly modified when, at 5 days after emergence, the flies were fed antibiotic-resistant bacteria belonging to two species commonly isolated from the gut of field-collected B. tryoni. Flies were fed one marked dinitrogen-fixing strain each of either Klebsiella oxytoca or Enterobacter cloacae, and the gastrointestinal tracts of fed flies were shown to be colonized within 7 days by antibiotic-resistant isolates of K. oxytoca but not E. cloacae. The composition of the microbial population also appeared to be stable in that the distribution and frequency of bacterial taxa among individual flies exhibited similar patterns whether or not the flies had been bacteria fed. Isolates of either E. cloacae or K. oxytoca, constituting 70% of the total numbers, were usually dominant, with oxidative species including pseudomonads forming the balance of the population. Antibiotic-resistant bacteria could be spread from one cage of flies to the adjacent surfaces of a second cage within a few days and had reached a control group several meters distant by 3 weeks. Restriction of marked bacteria to the population of one in five flies sampled from the control group over the next 30 days suggested that the bacterial population in the gut of the insect was susceptible to alteration in the first week after emergence but that thereafter it entered a steady state and was less likely to be perturbed by the introduction of newly encountered strains. All populations sampled, including controls, included at least one isolate of the dinitrogen-fixing family Enterobacteriaceae; many were distinct from the marked strains fed to the flies. Nitrogenase activity detected by the acetylene reduction assay was associated with flies fed dinitrogen-fixing bacteria as well as with control groups given either no supplement or free access to a yeast hydrolysate preparation. Nitrogen fixed from the atmosphere may supplement the nutrition of the alimentary tract microbial population of B. tryoni. Transmission electron microscopy showed that the principal site of bacterial colonization in the abdominal alimentary tract was the lumen of the midgut inside the peritrophic membrane. No intracellular symbionts were seen in the gut tissues nor were bacteria found attached to the cuticular folds of the hindgut. The ultrastructure of the gut resembled that of other fly genera except that the intercellular spaces between rectal epithelial cells were more extensive, suggesting a role for unspecialized epithelium in water and solute uptake in B. tryoni.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bignell D. E., Oskarsson H., Anderson J. M. Distribution and abundance of bacteria in the gut of a soil-feeding termite Procutiermes aburiensis (Termitidae, Termitinae). J Gen Microbiol. 1980 Apr;117(2):393–403. doi: 10.1099/00221287-117-2-393. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Pankratz H. S. In situ morphology of the gut microbiota of wood-eating termites [Reticulitermes flavipes (Kollar) and Coptotermes formosanus Shiraki]. Appl Environ Microbiol. 1977 Feb;33(2):406–426. doi: 10.1128/aem.33.2.406-426.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daddow L. Y. An abbreviated method of the double lead stain technique. J Submicrosc Cytol. 1986 Jan;18(1):221–224. [PubMed] [Google Scholar]
- Danso S. K., Habte M., Alexander M. Estimating the density of individual bacterial populations introduced into natural ecosytems. Can J Microbiol. 1973 Nov;19(11):1450–1451. doi: 10.1139/m73-234. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Tormey J. M. Role of long extracellular channels in fluid transport across epithelia. Nature. 1966 May 21;210(5038):817–820. doi: 10.1038/210817a0. [DOI] [PubMed] [Google Scholar]
- GREENBERG B. Persistence of bacteria in the developmental stages of the housefly. IV. Infectivity of the newly emerged adult. Am J Trop Med Hyg. 1959 Nov;8:618–622. doi: 10.4269/ajtmh.1959.8.618. [DOI] [PubMed] [Google Scholar]
- Harder W., Kuenen J. G. A review. Microbial selection in continuous culture. J Appl Bacteriol. 1977 Aug;43(1):1–24. doi: 10.1111/j.1365-2672.1977.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Bousch G. M., Baerwald R. J. Amino acid synthesis by Pseudomonas melophthora, bacterial symbiote of Rhagoletis pomonella (Diptera). J Insect Physiol. 1968 Apr;14(4):513–518. doi: 10.1016/0022-1910(68)90066-8. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Wessing A., Eichelberg D. Elektronenmikroskopische Untersuchungen zur Struktur und Funktion der Rektalpapillen von Drosophila melanogaster. Z Zellforsch Mikrosk Anat. 1973;136(3):415–432. [PubMed] [Google Scholar]