Abstract
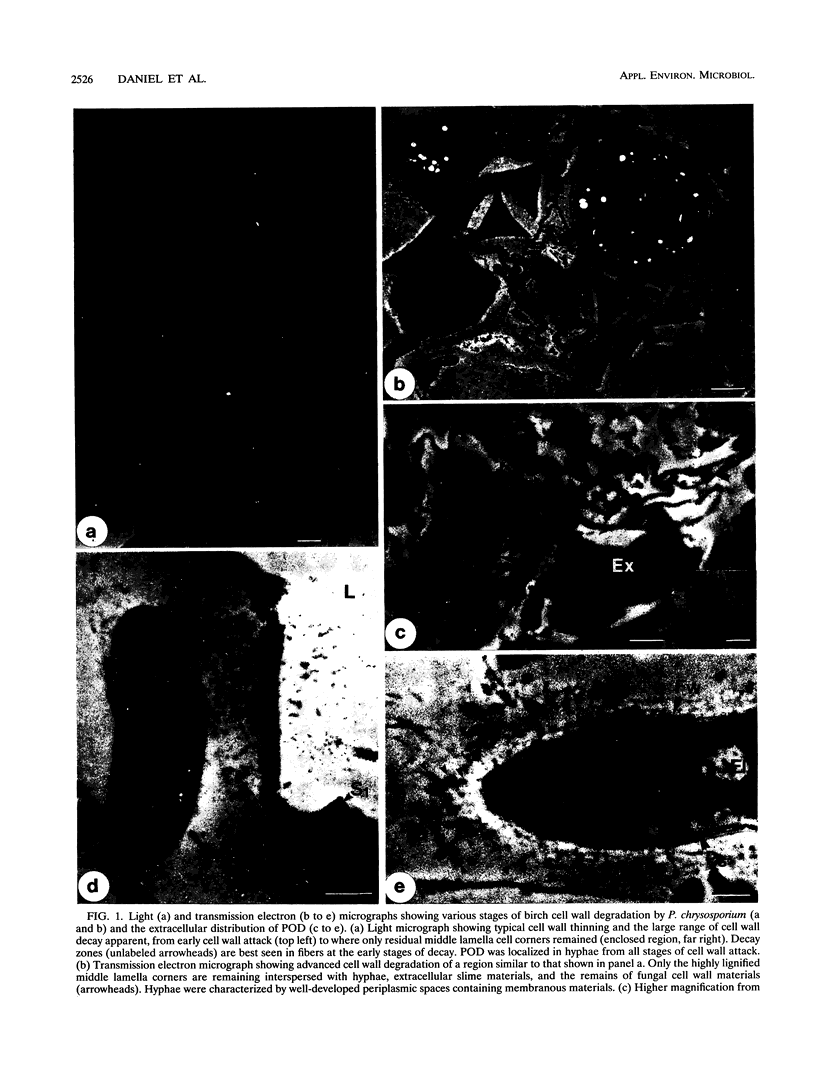
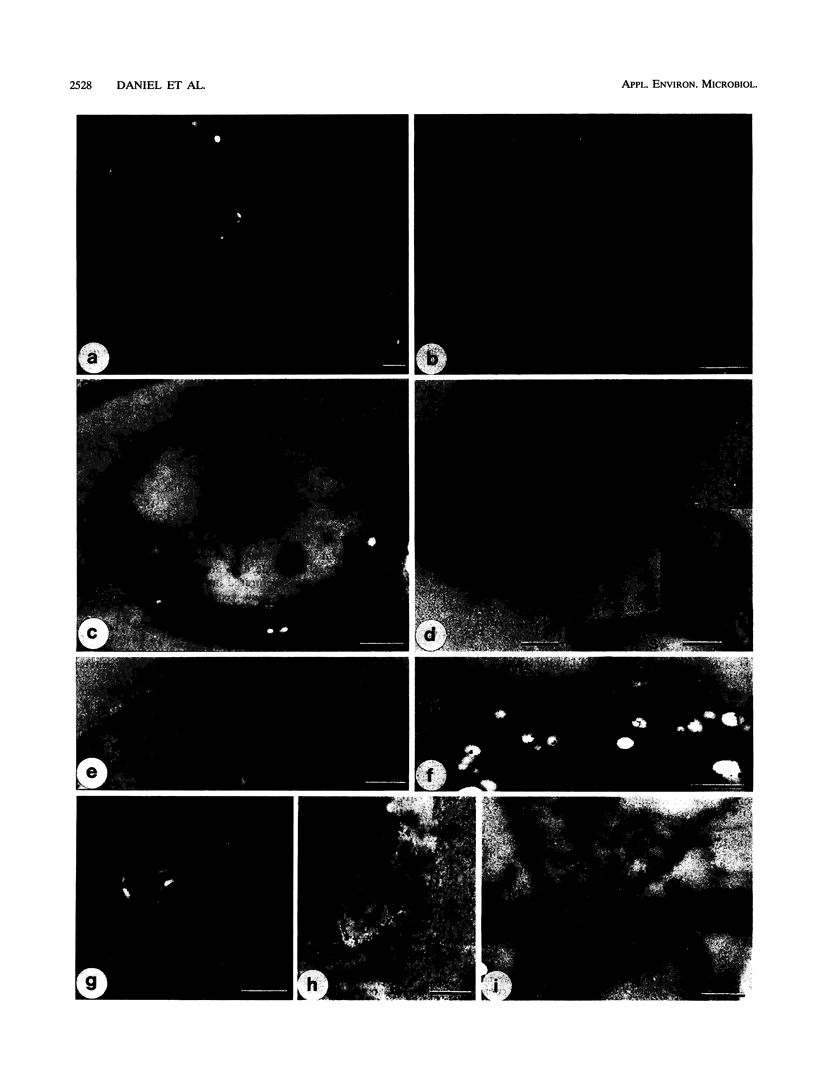
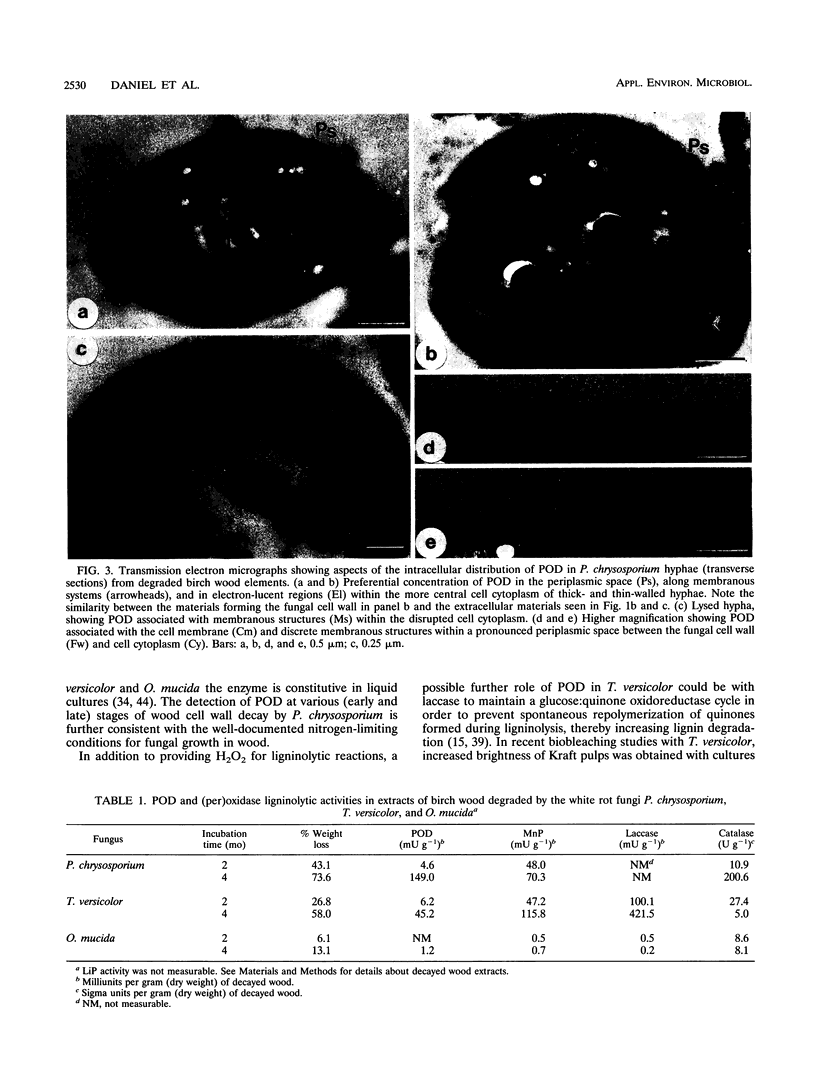
The production of the H2O2-generating enzyme pyranose oxidase (POD) (EC 1.1.3.10) (synonym, glucose 2-oxidase), two ligninolytic peroxidases, and laccase in wood decayed by three white rot fungi was investigated by correlated biochemical, immunological, and transmission electron microscopic techniques. Enzyme activities were assayed in extracts from decayed birch wood blocks obtained by a novel extraction procedure. With the coupled peroxidase-chromogen (3-dimethylaminobenzoic acid plus 3-methyl-2-benzothiazolinone hydrazone hydrochloride) spectrophotometric assay, the highest POD activities were detected in wood blocks degraded for 4 months and were for Phanerochaete chrysosporium (149 mU g [dry weight] of decayed wood-1), Trametes versicolor (45 mU g-1), and Oudemansiella mucida (1.2 mU g-1), corresponding to wood dry weight losses of 74, 58, and 13%, respectively. Mn-dependent peroxidase activities in the same extracts were comparable to those of POD, while lignin peroxidase activity was below the detection limit for all fungi with the veratryl alcohol assay. Laccase activity was high with T. versicolor (422 mU g-1 after 4 months), in trace levels with O. mucida, and undetectable in P. chrysosporium extracts. Evidence for C-2 specificity of POD was shown by thin-layer chromatography detection of 2-keto-d-glucose as the reaction product. By transmission electron microscopy-immunocytochemistry, POD was found to be preferentially localized in the hyphal periplasmic space of P. chrysosporium and O. mucida and associated with membranous materials in hyphae growing within the cell lumina or cell walls of partially and highly degraded birch fibers. An extracellular distribution of POD associated with slime coating wood cell walls was also noted. The periplasmic distribution in hyphae and extracellular location of POD are consistent with the reported ultrastructural distribution of H2O2-dependent Mn-dependent peroxidases. This fact and the dominant presence of POD and Mn-dependent peroxidase in extracts from degraded wood suggest a cooperative role of the two enzymes during white rot decay by the test fungi.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S. Lignin Peroxidase Activity Is Not Important in Biological Bleaching and Delignification of Unbleached Kraft Pulp by Trametes versicolor. Appl Environ Microbiol. 1992 Sep;58(9):3101–3109. doi: 10.1128/aem.58.9.3101-3109.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbonnais R., Paice M. G. Veratryl alcohol oxidases from the lignin-degrading basidiomycete Pleurotus sajor-caju. Biochem J. 1988 Oct 15;255(2):445–450. doi: 10.1042/bj2550445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel G., Nilsson T., Pettersson B. Intra- and Extracellular Localization of Lignin Peroxidase during the Degradation of Solid Wood and Wood Fragments by Phanerochaete chrysosporium by Using Transmission Electron Microscopy and Immuno-Gold Labeling. Appl Environ Microbiol. 1989 Apr;55(4):871–881. doi: 10.1128/aem.55.4.871-881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel G., Volc J., Kubatova E., Nilsson T. Ultrastructural and Immunocytochemical Studies on the H(2)O(2)-Producing Enzyme Pyranose Oxidase in Phanerochaete chrysosporium Grown under Liquid Culture Conditions. Appl Environ Microbiol. 1992 Nov;58(11):3667–3676. doi: 10.1128/aem.58.11.3667-3676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danneel H. J., Rössner E., Zeeck A., Giffhorn F. Purification and characterization of a pyranose oxidase from the basidiomycete Peniophora gigantea and chemical analyses of its reaction products. Eur J Biochem. 1993 Jun 15;214(3):795–802. doi: 10.1111/j.1432-1033.1993.tb17982.x. [DOI] [PubMed] [Google Scholar]
- Datta A., Bettermann A., Kirk T. K. Identification of a specific manganese peroxidase among ligninolytic enzymes secreted by Phanerochaete chrysosporium during wood decay. Appl Environ Microbiol. 1991 May;57(5):1453–1460. doi: 10.1128/aem.57.5.1453-1460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Pankratz H. S. Ultrastructural Localization of Hydrogen Peroxide Production in Ligninolytic Phanerochaete chrysosporium Cells. Appl Environ Microbiol. 1982 Sep;44(3):732–736. doi: 10.1128/aem.44.3.732-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. R. Significance of glucose oxidase in lignin degradation. Nature. 1977 Jul 7;268(5615):78–80. doi: 10.1038/268078a0. [DOI] [PubMed] [Google Scholar]
- Kelley R. L., Reddy C. A. Purification and characterization of glucose oxidase from ligninolytic cultures of Phanerochaete chrysosporium. J Bacteriol. 1986 Apr;166(1):269–274. doi: 10.1128/jb.166.1.269-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten P. J., Kirk T. K. Involvement of a new enzyme, glyoxal oxidase, in extracellular H2O2 production by Phanerochaete chrysosporium. J Bacteriol. 1987 May;169(5):2195–2201. doi: 10.1128/jb.169.5.2195-2201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocourek J., Tichá M., Kostír J. The use of diphenylamine-alanine-phosphoric acid reagent in the detection and differentiation of monosaccharides and their derivatives on paper chromatograms. J Chromatogr. 1966 Sep;24(1):117–124. doi: 10.1016/s0021-9673(01)98109-9. [DOI] [PubMed] [Google Scholar]
- Kremer S. M., Wood P. M. Production of Fenton's reagent by cellobiose oxidase from cellulolytic cultures of Phanerochaete chrysosporium. Eur J Biochem. 1992 Sep 15;208(3):807–814. doi: 10.1111/j.1432-1033.1992.tb17251.x. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volc J., Denisova N. P., Nerud F., Musílek V. Glucose-2-oxidase activity in mycelial cultures of basidiomycetes. Folia Microbiol (Praha) 1985;30(2):141–147. doi: 10.1007/BF02922207. [DOI] [PubMed] [Google Scholar]
- Volc J., Sedmera P., Musílek V. Glucose-2-oxidase activity and accumulation of D-arabino-2-hexosulose in cultures of the basidiomycete Oudemansiella mucida. Folia Microbiol (Praha) 1978;23(4):292–298. doi: 10.1007/BF02876683. [DOI] [PubMed] [Google Scholar]