Abstract
Background:
Transforming growth factor-β1 (TGF-β1), a key biological mediator following ionizing radiation, plays a role in a complex tissue reaction involved in local radiation-induced pathological damage. Knocking out Smad3 (S3KO), a downstream signaling intermediate in the TGF-β pathway, in mice protects their skin from radiation damage as demonstrated by decreased epithelial acanthosis and dermal fibrosis as compared to Smad3 wild-type (S3WT) mice.
Objective:
The present study was designed to investigate the molecular mechanisms contributing to increased radioprotection in the absence of Smad3.
Methods:
Primary dermal fibroblasts derived from S3WT and KO mice were exposed to 5Gy ionizing radiation in vitro. Western blot analyses, immunocytochemistry, and reporter transfections were used to dissect the radiation-induced events.
Results:
There was increased phosphorylation of ERK-MAPK, p53 and H2A.X in S3KO compared to the S3WT fibroblasts, implicating them in a key signaling cascade in response of these cells to radiation. Pro-fibrotic gene expression was decreased in S3KO fibroblasts post-irradiation.
Conclusion:
The absence of Smad3 may decrease radio-responsiveness by increasing activation of DNA damage sensing mechanisms and decreasing induction of pro-fibrotic genes.
1. Introduction
Transforming growth factor β (TGF-β) has been shown to be central to radiation-induced cytokine responses in vivo, including a prominent role in both the profuse synthesis and reorganization of matrix, as well as the increased apoptosis of epithelial and hematopoetic cells present in the targeted radiation field [1, 2]. Tissue irradiation damage involve both the primary biochemical and physiological events that occur immediately following ionizing irradiation, as well as secondary effects that are seen days, months or even years after the initial exposure. Studies in TGF-β1 null mice and attempts to neutralize TGF-β have highlighted its role in both primary and secondary cellular and tissue damage induced by ionizing radiation [3].
The predominant post-radiation damage in skin of Smad3 WT (S3WT) mice is increased thickness of the epithelial spinous layer (acanthosis) and pronounced dermal fibrosis, characterized by both increased deposition and disorganized architecture of collagens [4]. We had shown previously that the Smad3 KO (S3KO) mice are less responsive to local cutaneous radiation demonstrating decreased acanthosis and minimal fibrosis [4], while also demonstrating improved healing post-irradiation [5], a response that can be compromised in patients undergoing radiotherapy.
Fibroblasts are a key cellular target in mediating tissue radiation responses both in terms of stromal responses, as well as paracrine modulation of other cell lineages including epithelial cells and infiltrating and resident inflammatory cells. The key determinant of radio-responsiveness seems to be the cell's ability to detect and repair DNA damage while the initiation of various intra- and extracellular signaling networks orchestrate the eventual outcome at the tissue and organismal levels [3].
In this study we used primary dermal fibroblasts isolated from S3 WT and KO mice as a simplified in vitro system to begin to dissect the possible molecular signaling cascades involved in the observed radioprotection seen in the S3 KO mice.
2. Materials and Methods
2.1 Primary Cell Culture
Primary dermal fibroblasts were isolated from S3WT and S3KO one-day old pups as previously described [4]. Cells were propagated in DMEM (GIBCO, Grand Island, NY), 10% FBS (Hyclone, Logan, UT), 1% Penstrep (Gibco) and used semi-confluent between 2-7 passages for all assays.
2.2 Chemicals and reagents
The following inhibitors or vehicles (DMSO/PBS) were added 30-60 min prior to treatment: SB431542 (Alk5) 5 μM (Sigma, St. Louis, MO); PD98059 (ERK) 25 μM; SB202190 (p38) 10 μM; and SP600125 (JNK) 10 μM (Calbiochem, San Diego, CA).
2.3 Ionizing irradiation
Primary dermal fibroblasts were trypsinized and 1 × 106 cells were plated in 10% serum in 10 cm dishes and allowed to attach overnight before being starved for 10 h in 0.2% serum. Cells were then exposed to γ-irradiation (5 Gy) from a 60Co source, maintained in 0.2% serum for up to 24 h for signaling experiments, and then lysed at particular time points.
2.4 Transfections
Fibroblasts were plated in 6 well plates (3 × 104 cells/well) and co-transfected with CAGA-12, ARE-FAST1, pT3P (PAI), AP1, c-Jun and COL1A2-CAGA-TK-luc (courtesy of John Varga, University of Illinois at Chicago) reporters along with pRLTK-luciferase using Fugene6 (Roche, Nutley, NJ). Cells were allowed to recover overnight, serum-starved for 10 h before being irradiated (5 Gy). After an additional 16-18 h, cells were lysed in passive lysis buffer (Promega, Madison, WI) and assayed with the dual luciferase kit as per manufacturer's instructions (Promega).
2.5 Immunoblot Analyses
Immunoblotting was performed as described previously [6]. Briefly, cells were scraped into lysis buffer (HEPES pH 7.4 (25mM), sodium chloride (175mM), glycerol (0.1%), EDTA (5mM), Triton X-100 (0.1%), sodium fluoride (50mM), sodium orthovanadate (5mM), complete mini protease inhibitor cocktail (Roche)) and spun at 14000 rpm for 15 min to clear cellular debris. Total protein was estimated by BCA assay (Pierce, Rockford, IL). Samples (20-50 μg) were then mixed with loading buffer and reducing agents (both Invitrogen, Carlsbad, CA) and heated at 72°C for 10 min followed by cooling on ice, spinning at 14000 rpm for 2 min, running on pre-cast Tris-Glycine gels (Invitrogen), and transferring onto 0.2 μm nitrocellulose membranes (GE Healthcare, Piscataway, NJ). Blots were incubated with primary antibodies total and phospho-JNK, total and phospho-ERK, phospho-p53 (serine 15), phospho-MDM2 (all from Cell Signaling, Danvers, MA), phospho-Smad3 (kind gift from Ed Leof, Mayo Clinic, Rochester, MN), total and phospho-H2A.X, HSP-70 (both Millipore, Charlottesville, VA), p53 (Ab-5, Lab Vision Corp., Fremont, CA) and β-actin (Chemicon, Temecula, CA) followed by appropriate species-specific secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Chemiluminescence (Pierce) was detected by films (MR Kodak, Rochester, NY) or digital imager (Alpha Inotech, San Leandro, CA).
H2A.X analyses
Staining for γH2A.X was carried out as described previously [7]. Briefly, cells were washed with Tris-buffered saline (TBS) and fixed-permeabilized with 95% ethanol and 5% acetic acid for 5min, washed with TBS, blocked with 3% BSA-TBS and incubated with 1:2500 anti-phospho-H2A.X (Millipore) for 1hr at room temperature. Following washes with TBS, secondary FITC-conjugated antibody was added for 1.5 h, and slides were washed with TBS and mounted with mounting medium containing DAPI (Vector labs).
3. Results
3.1 Decreased transcriptional activity in S3KO cells following irradiation
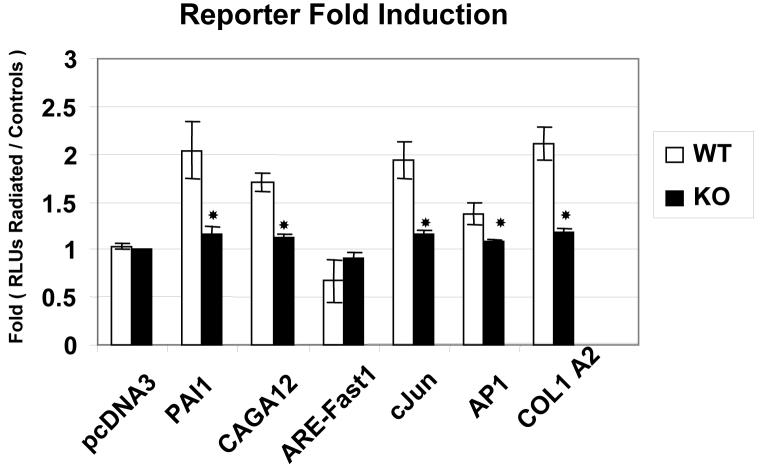
Since skin of S3 KO mice showed less epithelial damage and extracellular matrix synthesis following exposure to ionizing irradiation than did skin of WT mice, we hypothesized that S3 KO cells might be less responsive to radiation. We examined this using reporter constructs of collagen1α2 and common AP1 reporters to determine if their downstream transactivations were differentially modulated by the Smad3 status in these primary fibroblasts following irradiation. Transfections with the Smad3 specific reporter CAGA-12 and a pan-TGF-β1 reporter (PAI1) demonstrated that the TGF-β1 pathway was indeed activated by irradiation in the S3WT cells (p<0.05), but was deficient in the S3KO cells (Fig. 1). The Smad2 specific reporter (ARE / FAST1) was not significantly affected in either cell line following irradiation. We observed decreased activation of both c-Jun and AP1 reporters in S3KO cells as compared to S3WT cells (Fig.1), suggesting decreased radio-responsiveness. The activation of the collagen1α2-AP reporter in S3WT cells following radiation was clearly deficient in the S3KO cells (p<0.05) supporting the decreased fibrosis observed in vivo in the S3KO mice [4].
Figure 1.
Gene activation following irradiation. Primary dermal fibroblasts were transfected with various gene specific reporters to demonstrate differences in their activation as affected by Smad3 status namely, PAI1 (Plasminogen activator inhibitor 1) a ‘Pan’ TGF-β reporter; CAGA12 (Smad3-specific); ARE-FAST1 (Smad2-specific); c-Jun, AP1 and collagen1β2 (radiation response induced reporters). Fold-induction over basal reporter activity is represented as relative luciferase units (RLU) and is representative of at least 3 independent experiments, * p<0.05.
3.2 Similar cell growth of S3 WT and KO dermal fibroblasts following irradiation
We began to look for differences in radiation responsiveness in S3 WT and KO dermal fibroblasts by comparing cell growth characteristics after exposure to ionizing radiation. Both cell types demonstrated similar numbers of viable cells when maintained in either full (10%) or low (0.2%) serum for up to 5 days following irradiation. (Data not shown and [4]). Additionally, FACS analyses showed that cells transited through the cell cycle similarly at 24 h post-irradiation.
3.3 Enhanced ERK-MAPK activation in S3KO cells following ionizing radiation
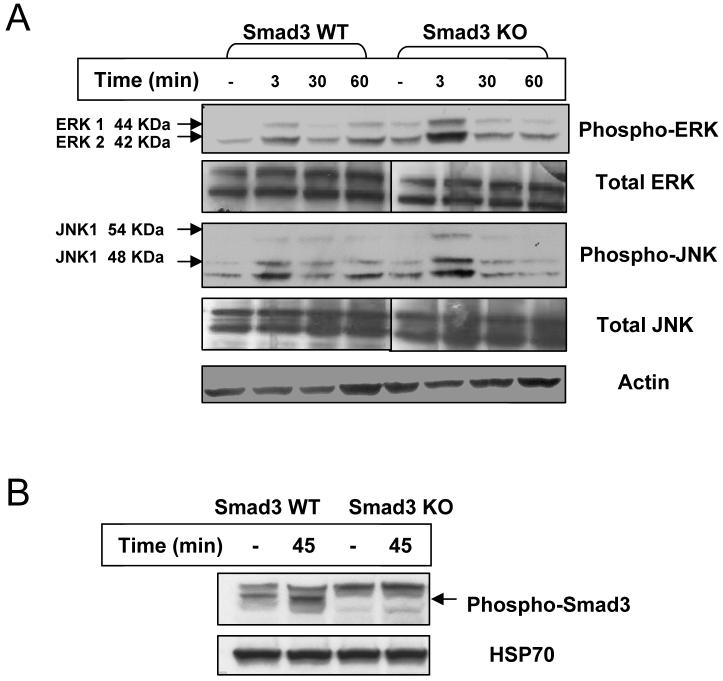
Following exposure to ionizing irradiation, an increase of phospho-ERK MAPK in S3KO and S3WT primary fibroblasts was observed (Fig. 2A). While an increase in phospho-ERK levels occurred with similar kinetics in both cell types, the activation in S3KO cells appeared to be more pronounced. Interestingly, a similar increase in phospho-JNK MAPK levels in S3KO cells compared to S3WT fibroblasts was also observed, while phospho-p38 MAPK did not seem to be affected in the S3KO fibroblasts (Fig. 2A and data not shown). Total levels of these proteins were not affected following irradiation (Fig. 2A). Activation of the TGF-β pathway by irradiation as evidenced by increased phospho-Smad3 in the S3WT fibroblasts was seen (Fig. 2B).
Figure 2.
Radiation-activated MAPK and TGF-β signaling pathways. Primary dermal fibroblasts derived from Smad3 WT and KO mice in monolayer semiconfluent cultures were irradiated with 60Co with a total dose 5 Gy and lysed. Immunoblot analysis was performed for (A) total and phospho-ERK and JNK MAPK and (B) phospho-Smad3. Blots are representative of 3 independent experiments.
3.4 Serine 15 phosphorylation of p53 is increased in S3KO cells via the ERK-MAPK pathway
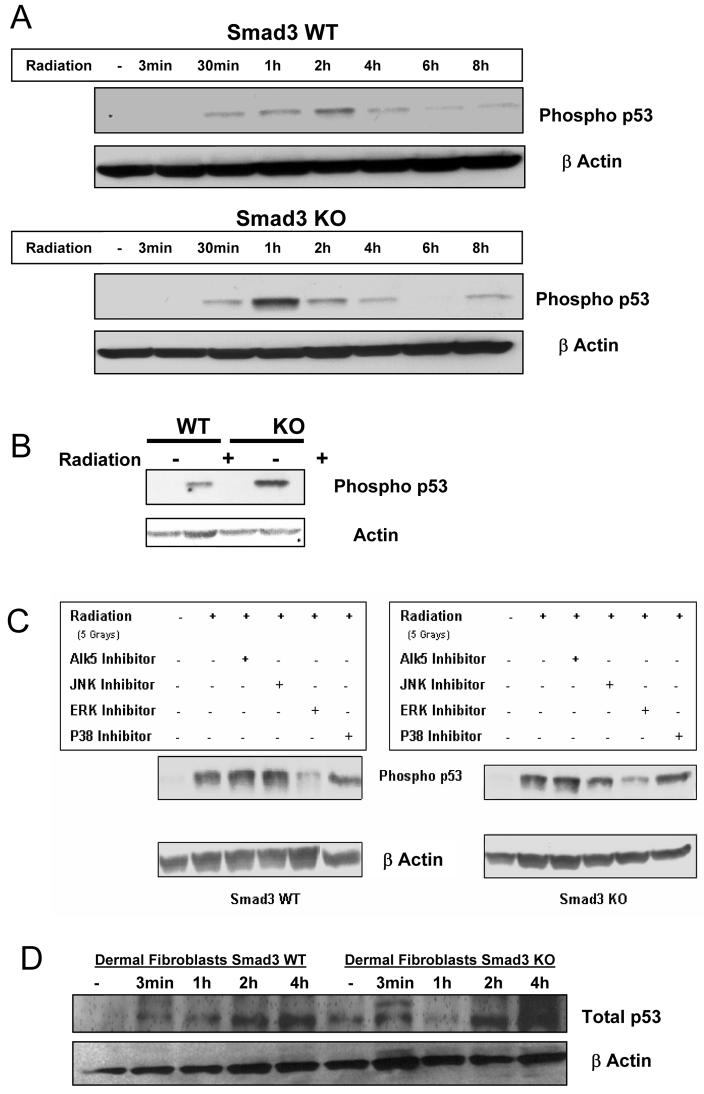
The phosphorylation of serine 15 of p53 specifically impairs its ability to interact with MDM2, promoting its accumulation and function in response to DNA damage [8]. To determine if phospho-p53 status might contribute to reduced radio-responsiveness in S3 KO dermal fibroblasts, we examined levels of phosphoSer-15 in S3WT and KO cells at various times post-irradiation. Both S3WT and KO cells showed increased phospho-p53 at 30 min post-irradiation, but by 1 h the S3KO cells showed 5-fold greater phospho-p53 than did WT cells when compared to non-irradiated cells (Fig. 3A). In both cell types the expression then decreased though 8h. A direct comparison of the phospho-p53 at the 1h time points is shown in Fig 3B.
Figure 3.
(A) Increased p53 phosphorylation in S3 KOs following radiation. Smad3WT and KO primary dermal fibroblasts were irradiated and cells lysed at specific time points for immunoblot analyses and probed for phospho p53 at serine 15. Blots are representative of 3 independent experiments. (B) Direct comparison of induction of phospho-p53 at 1 h post-irradiation in S3WT and KO dermal fibroblasts. (C) Pathway specific inhibitors used to dissect the role of MAPK in p53 phosphorylation. Smad3WT and KO primary dermal fibroblasts were pretreated with inhibitors followed by irradiation and 1 h later were assayed for phospho-p53 status by immunoblotting. Blots are representative of 3 independent experiments. (D) Levels of total p53 in S3 WT and KO dermal fibroblasts at later times post-irradiation.
MAPKs are activated following exposure to ionizing radiation (Fig. 2A) and they can directly affect the phosphorylation of p53 at multiple sites [9, 10]. The specific signaling cascade involved in this response was explored by using inhibitors specific to the ERK, JNK, p38 and TGF-β signaling pathways. Our data demonstrate the central role of ERK-MAPK in modulating the specific phosphorylation of p53 at serine 15, while the other inhibitors do not affect this specific event (Fig. 3C). The inability of the ALK5 inhibitor to affect the serine 15 phosphorylation of p53 indicates the absence of a direct role of TGF-β in modulating this particular cellular radiation response in both S3WT and S3KO cells.
Interestingly, both cell lines demonstrate increases in total p53 levels following ionizing irradiation which occurred at later time points than as observed with increases in phospho-p53 (Fig. 3D). This increase seemed more apparent in the S3KO DFs at 4 h. This data is consistent with the observation that some of the increased phosphoserine 15 activated p53 levels in S3KO cells might also result from increased p53 transactivation as has been previously reported [11, 12].
3.5 Increased Phosho-H2A.X in S3KO fibroblasts following irradiation
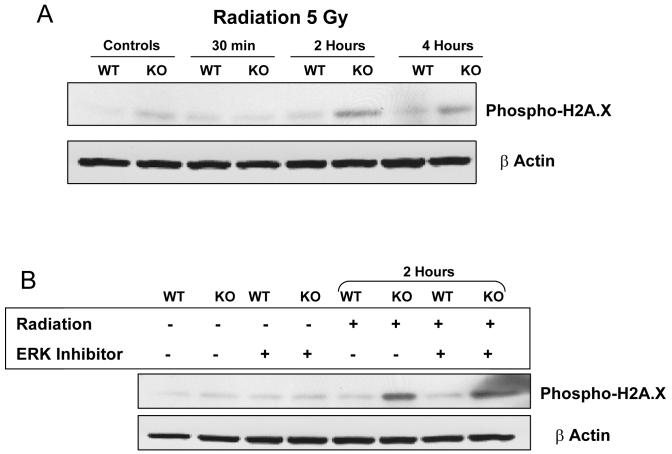
Irradiated S3KO dermal fibroblasts appear to have an exaggerated cellular response to irradiation compared to the S3WT cells as evidenced by enhanced activation of both MAPK and p53. This differential response is further supported by the levels of increased phospho-Histone H2A.X in the S3KO cells compared to S3WT cells at 4 h post-irradiation as determined by immunofluorescence (data not shown). Phosphorylation of histone H2A.X at serine 139 represents DNA damage sensing both in terms of damage due to double strand breaks, as well as to the repair intermediates initiated due to this damage [13]. This increase in phospho-H2A.X levels occurred earlier and to a greater extent in S3KO than in S3WT cells (Fig. 4A), while total levels of H2A.X were not altered following irradiation (data not shown). The increased levels in S3KO cells were not affected by the ERK inhibitor suggesting that the p53 and H2A.X phosphorylation responses are modulated by different upstream signaling pathways (Fig. 4B). The increased phospho-H2A.X levels suggest that these cells, in the absence of Smad3, might have a more exquisite DNA damage sensing, repair or bypass mechanism in response to radiation.
Figure 4.
Phospho-H2A.X analyses for DNA damage sensing-repair. Primary dermal fibroblasts were plated in culture dishes, irradiated, and allowed to recover for various times. (A) Cells were lysed and immunoblotted for phsopho-H2A.X. (B) At the 2 h time point cells were cultured in the absence or presence of the ERK inhibitor. Blots are representative of 2 experiments.
4. Discussion
Ionizing radiation activates multiple intracellular pathways critical to controlling cell survival and repopulation in a stress-specific, microenvironment-modulated and cell type-dependent manner. There are often inter-connections or crosstalk at various levels between these pathways. As eloquently outlined in a recent review, biological systems are usually modeled as hierarchical, linear responses rather than combinatorial networks [3]. Among these intricate signaling networks, several major pathways are evoked by radiation including PI3K, MAPKs, and ATM that are mediated through a variety of growth factor and cytokine membrane receptors [14, 15]. The TGF-β signal transduction pathway functions through heterodimeric serine-threonine kinase receptors, TβRII and TβRI (Alk5) and nuclear-cytoplasmic signaling intermediates, predominantly Smads 2, 3, 4, and 7 [16]. A significant role for other TGF-β-dependent signaling pathways, referred to as ‘lateral signaling’, through MAPK and PI3K have also been documented. ERK-MAPK has been shown to directly affect Smad3 phosphorylation and vice versa, implicating the intimate molecular association of these pathways downstream of ligand-activated signaling [17].
We show in this study that the TGF-β pathway is activated post-irradiation as evidenced by activation of TGF-β specific promoters (Fig. 1) and the phosphorylation of Smad 3 (Fig. 2). The absence of Smad3 in primary dermal fibroblasts more effectively induces ERK-MAPK mediated damage-repair sensing molecular cascades such as the phosphorylation and transcriptional induction of p53 (Fig. 3) and phosphorylation of H2A.X (Fig. 4) that could contribute to the radioprotection observed in the Smad3 null mice. We did not observe any significant decrease in phospho-p53 using the Alk5 inhibitor, implicating a TGF-β independent, but Smad-dependent event mediated by ERK-MAPK, as has been shown recently in other systems [17]. The upstream ligand-receptor complex inducing ERK-MAPK activation post-irradiation, as well as activation of other signaling intermediates such as Raf, Ras, intracellular calcium and reactive oxygen species remains to be investigated.
The initial physical and chemical events following external radiation energy deposition are similar in any given cell population and differences in responsiveness are largely determined by the ability of individual cells to respond to and to repair DNA damage. The primary response to radiation in hematopoietic cells is apoptosis, while fibroblasts undergo growth arrest, and epithelial cells can undergo either process depending on anatomical tissue of origin and degree of damage [2]. We did not see any significant differences in growth or apoptosis of S3WT and KO dermal fibroblasts following irradiation. A recent study demonstrated that the S3KO bone marrow stromal cell lines exhibit radioresistance as evidenced by the increased cell population in the G2 / M cell cycle phase, reduced migration and phenotypic changes [18]. However, we see no significant difference in survival of S3WT and KO mice following whole body irradiation [4], suggesting that loss of Smad 3 does not afford protection from bone marrow toxicity. Additionally, our experiments with bone marrow swapping between the S3WT and S3KO mice failed to confer any significant protection to locally irradiated skin, suggesting these cells might have a more redundant role in radioprotection as compared to the local milieu (manuscript in preparation).
Phosphorylation of Histone H2A.X (γH2A.X) at serine-139 is an often used method to analyze DNA double strand break damage [7] and repair [11] and is widely used to predict radiosensitivity of cell lines [19]. Increased γH2A.X has been correlated with increased concentrations of repair factors [20] and its absence has been shown to be associated with genomic instability and radiation sensitivity [21]. The increased γH2A.X seen in the S3KO fibroblasts post-radiation may correlate with their decreased radio-responsiveness, while also directly implicating the ERK-MAPK pathway in its initiation. Phosphorylation and subsequent activation of p53, as well as its transcription induction, are key initial responses to DNA damage. The increased phospho-p53 observed in the S3KO cells, along with the increased phospho-H2A.X suggests these cells have a more robust DNA damage surveillance and possibly repair mechanism helping them either adapt to or overcome critical radiation-induced DNA damage. This may, in turn, imply that these cells in vivo might induce less paracrine or bystander damage as observed in S3KO irradiated skin [4].
TGF-β plays a central role in mediating both the acute (primary) and the long term (secondary) radiation responses in vivo. Immediately following irradiation, redox-mediated activation of latent TGF-β leading to Smad activation has been demonstrated [22, 23]. This induction of TGF-β may play a role in mediating the primary tissue response of the mesenchyme to ionizing radiation, which is the induction of matrix genes in an attempt by local cells to spatially restrict the damage. Indeed increased levels of TGF-β following radiation correlate with increased susceptibility to fibrosis in cancer patients [24-26], as well as in the lungs of various mouse strains [27]. The AP1 complex also has been shown to be directly modulated by radiation and TGF-β [28, 29] and is important for intracellular signaling in radio-responsiveness [30]. Our data with the AP1 reporters suggests that this downstream effector pathway is also compromised in the S3KO cells compared to the S3WT cells, thus reducing the radiation-induced gene expression especially of the pro-fibrotic matrix genes and eventual tissue fibrosis [4, 31].
We demonstrate in this study that the altered radio-responsiveness in S3KO primary dermal fibroblasts through modulated molecular signaling cascades and DNA damage responses implicates a key role for Smad3 in mediating mesenchymal fibrosis in vivo. We have also analyzed primary keratinocytes derived from these mice in monolayer culture which did not show any significant difference in either signaling cascades or key radio-responsive markers (data not shown). The altered signaling in Smad3KO fibroblasts may also have indirect paracrine or bystander effects on other local cellular pools as has been shown for fibroblasts in tumor-stromal interactions [32, 33]. The radioprotection in Smad3 KO mice clearly demonstrates the potential efficacies of therapeutic intervention by blocking this pathway. However, unless the molecular events are precisely deciphered, the multifaceted effects of TGF-β and Smads on other non-target cell pools may be detrimental and therefore requires further exploration.
Acknowledgments
We thank Kelly Grimes for H2A.X staining, Ed Leof for phospho-Smad3 antibody, John Varga for COL1A2 reporter construct, Anastasia Sowers, Mario Anzano, Anthony Vieira and Larry Mullen for help with mice experiments. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996;34:251–66. doi: 10.1016/0360-3016(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 2.Hill RP, Rodemann HP, Hendry JH, Roberts SA, Anscher MS. Normal tissue radiobiology: from the laboratory to the clinic. Int J Radiat Oncol Biol Phys. 2001;49:353–65. doi: 10.1016/s0360-3016(00)01484-x. [DOI] [PubMed] [Google Scholar]
- 3.Barcellos-Hoff MH, Costes SV. A systems biology approach to multicellular and multigenerational radiation responses. Mutat Res. 2006;597:32–8. doi: 10.1016/j.mrfmmm.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Flanders KC, Sullivan CD, Fujii M, et al. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057–68. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flanders KC, Major CD, Arabshahi A, et al. Interference with transforming growth factor beta / Smad3 signaling results in accelerated healing of wounds in previously irradiated skin. Am J Pathol. 2003;163:2247–57. doi: 10.1016/s0002-9440(10)63582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arany PR, Flanders KC, Kobayashi T, Kuo CK, Stuelten C, Desai KV, Tuan R, Rennard SI, Roberts AB. Smad3 deficiency alters key structural elements of the extracellular matrix and mechanotransduction of wound closure. Proc Natl Acad Sci USA. 2006;103:9250–5. doi: 10.1073/pnas.0602473103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banath JP, Olive PL. Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Res. 2003;63:4347–50. [PubMed] [Google Scholar]
- 8.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–34. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 9.She QB, Chen N, Dong Z. ERKs amd p38 kinase phosphorylate p53 protein at serine 15 in response to UV radiation. J Biol Chem. 2000;275:20444–9. doi: 10.1074/jbc.M001020200. [DOI] [PubMed] [Google Scholar]
- 10.Sabatini N, DiGiacomo V, Rapino M, Rana R, Garaci G, Cataldi A. JNK/p53 mediated cell death response in K562 exposed to etoposide-ionizing radiation combined treatment. J Cell Biochem. 2005;95:611–9. doi: 10.1002/jcb.20392. [DOI] [PubMed] [Google Scholar]
- 11.Dumaz N, Meek DW. Serine15 phosphorylation stimulates p53 transactivation but does not directly influence interaction with HDM2. EMBO J. 1999;18:7002–10. doi: 10.1093/emboj/18.24.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert PF, Kashanchi F, Radonovich MF, Shiekhattar R, Brady JN. Phosphorylation of p53 Serine 15 increases interaction with CBP. J Biol Chem. 1998;273:33048–53. doi: 10.1074/jbc.273.49.33048. [DOI] [PubMed] [Google Scholar]
- 13.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–95. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 14.Dent P, Yacoub A, Fisher PB, Hagan M, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–96. doi: 10.1038/sj.onc.1206701. [DOI] [PubMed] [Google Scholar]
- 15.Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Byfield SD, Roberts AB. Lateral signaling enhances TGF-beta response complexity. Trends Cell Biol. 2004;14:107–11. doi: 10.1016/j.tcb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 18.Epperly MW, Goff JP, Zhang X, Niu Y, et al. Increased radioresistance, G2/M checkpoint inhibition, and impaired migration of bone marrow stromal cell lines derived from Smad3(− /−) mice. Radiat Res. 2006;165:671–7. doi: 10.1667/RR3572.1. [DOI] [PubMed] [Google Scholar]
- 19.Macphail SH, Banath JP, Yu TY, Chu EH, Lambur H, Olive PL. Expression of phosphorylated histone H2AX in cultured cell lines following exposure to X-rays. Int J Radiat Biol. 2003;79:351–8. doi: 10.1080/0955300032000093128. [DOI] [PubMed] [Google Scholar]
- 20.Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, Bonner RF, Bonner WM, Nussenzweig A. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol. 2003;5:675–9. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 21.Celeste A, Difilippantonio S, Difilippantonio MJ, et al. H2AX haploinsufficiency modifies genomic stability and tumor susceptibility. Cell. 2003;114:371–83. doi: 10.1016/s0092-8674(03)00567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrhart EJ, Segarini P, Tsang ML, Carroll AG, Barcellos-Hoff MH. Latent transforming growth factor beta1 activation in situ: quantitative and functional evidence after low-dose gamma-irradiation. FASEB J. 1997;11:991–1002. doi: 10.1096/fasebj.11.12.9337152. [DOI] [PubMed] [Google Scholar]
- 23.Ewan KB, Henshall-Powell RL, Ravani SA, Pajares MJ, Arteaga C, Warters R, Akhurst RJ, Barcellos-Hoff MH. Transforming growth factor-beta1 mediates cellular response to DNA damage in situ. Cancer Res. 2002;62:5627–31. [PubMed] [Google Scholar]
- 24.Li C, Wilson PB, Levine E, Barber J, Stewart AL, Kumar S. TGF-beta1 levels in pretreatment plasma identify breast cancer patients at risk of developing post-radiotherapy fibrosis. Int J Cancer. 1999;84:155–9. doi: 10.1002/(sici)1097-0215(19990420)84:2<155::aid-ijc11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Zheng H, Sung CC, Richter KK, Hauer-Jensen M. Cellular sources of transforming growth factor-beta isoforms in early and chronic radiation enteropathy. Am J Pathol. 1998;153:1531–40. doi: 10.1016/s0002-9440(10)65741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anscher MS, Murase T, Prescott DM, Marks LB, Reisenbichler H, Bentel GC, Spencer D, Sherouse G, Jirtle RL. Changes in plasma TGF beta levels during pulmonary radiotherapy as a predictor of the risk of developing radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1994;30:671–6. doi: 10.1016/0360-3016(92)90954-g. [DOI] [PubMed] [Google Scholar]
- 27.Franko AJ, Sharplin J, Ghahary A, Barcellos-Hoff MH. Immunohistochemical localization of transforming growth factor beta and tumor necrosis factor alpha in the lungs of fibrosisprone and “non-fibrosing” mice during the latent period and early phase after irradiation. Radiat Res. 1997;147:245–56. [PubMed] [Google Scholar]
- 28.Li Z, Xia L, Lee LM, Khaletskiy A, Wang J, Wong JU, Li JJ. Effector genes altered in MCF-7 human breast cancer cells after exposure to fractionated ionizing radiation. Radiat Res. 2001;155:543–53. doi: 10.1667/0033-7587(2001)155[0543:egaimh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 29.Hall MC, Young DA, Waters JG, Rowan AD, Chantry A, Edwards DR, Clark IM. The comparative role of activator protein 1 and Smad factors in the regulation of Timp-1 and MMP-1 gene expression by transforming growth factor-beta 1. J Biol Chem. 2003;278:10304–13. doi: 10.1074/jbc.M212334200. [DOI] [PubMed] [Google Scholar]
- 30.Avantaggiati ML, Ogryzko V, Gardner K, Giordano A, Levine AS, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–84. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh AK, Bhattacharyya S, Varga J. The tumor suppressor p53 abrogates Smad-dependent collagen gene induction in mesenchymal cells. J Biol Chem. 2004;279:47455–63. doi: 10.1074/jbc.M403477200. [DOI] [PubMed] [Google Scholar]
- 32.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–60. [PubMed] [Google Scholar]
- 33.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]