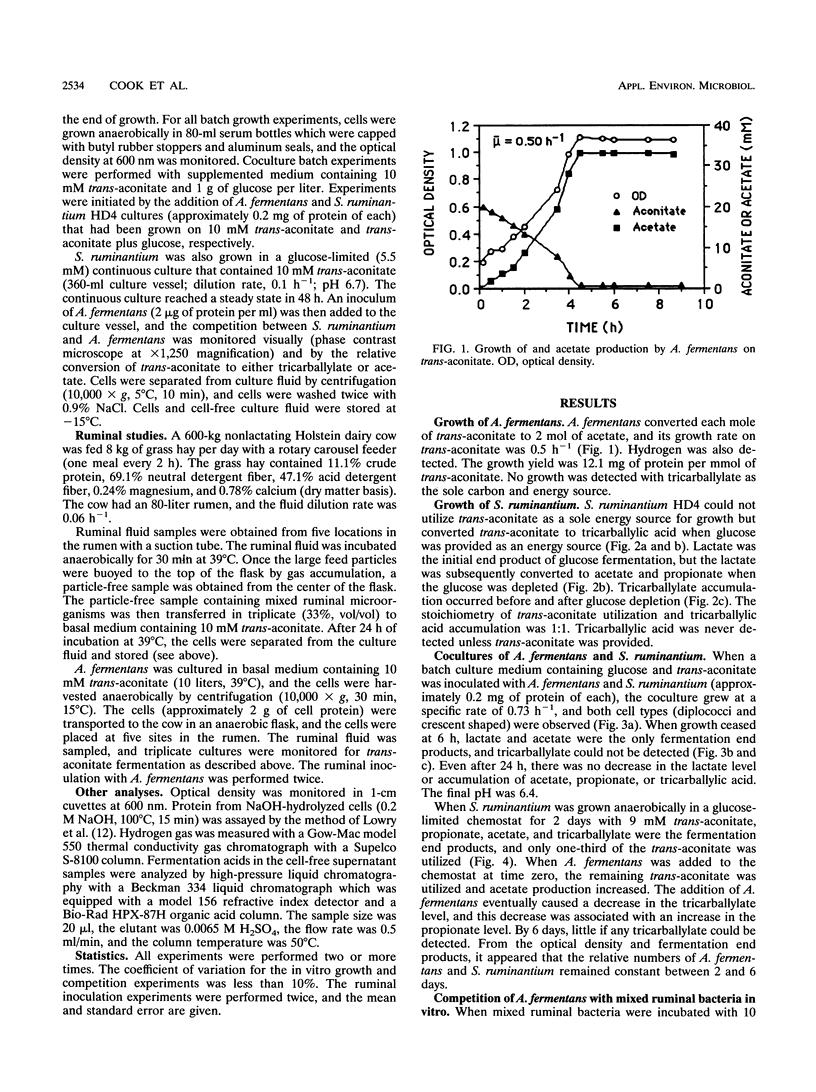
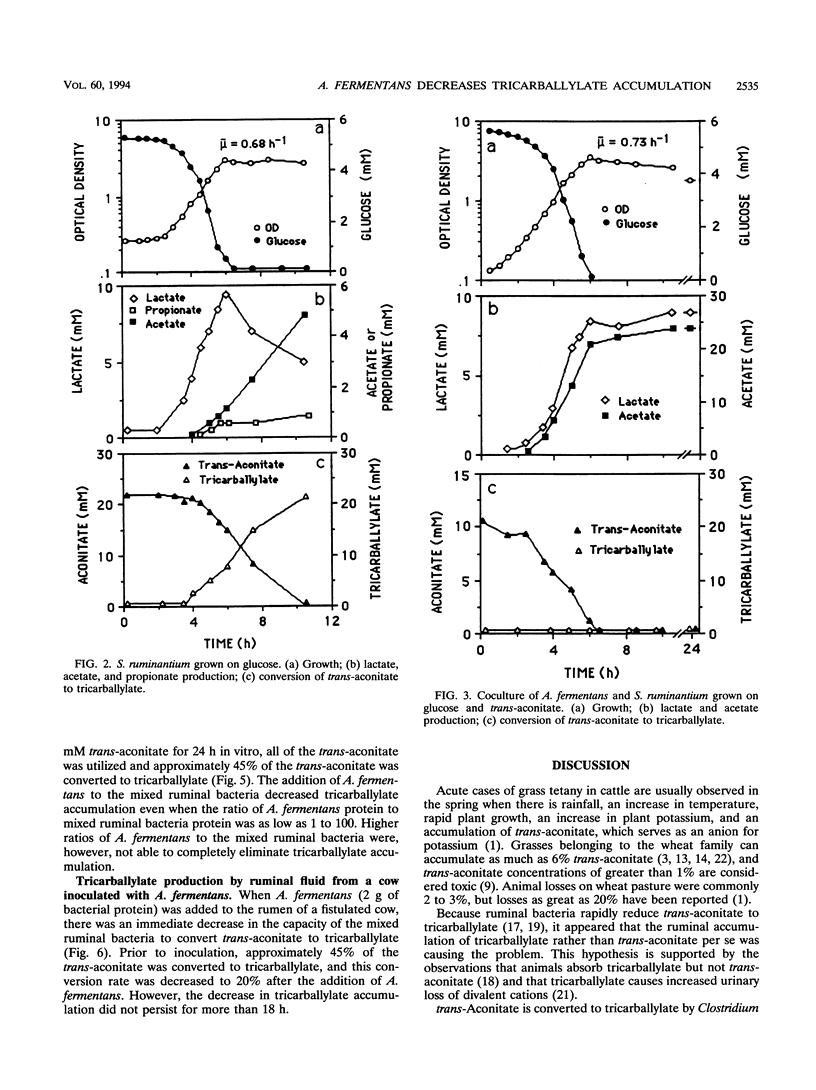
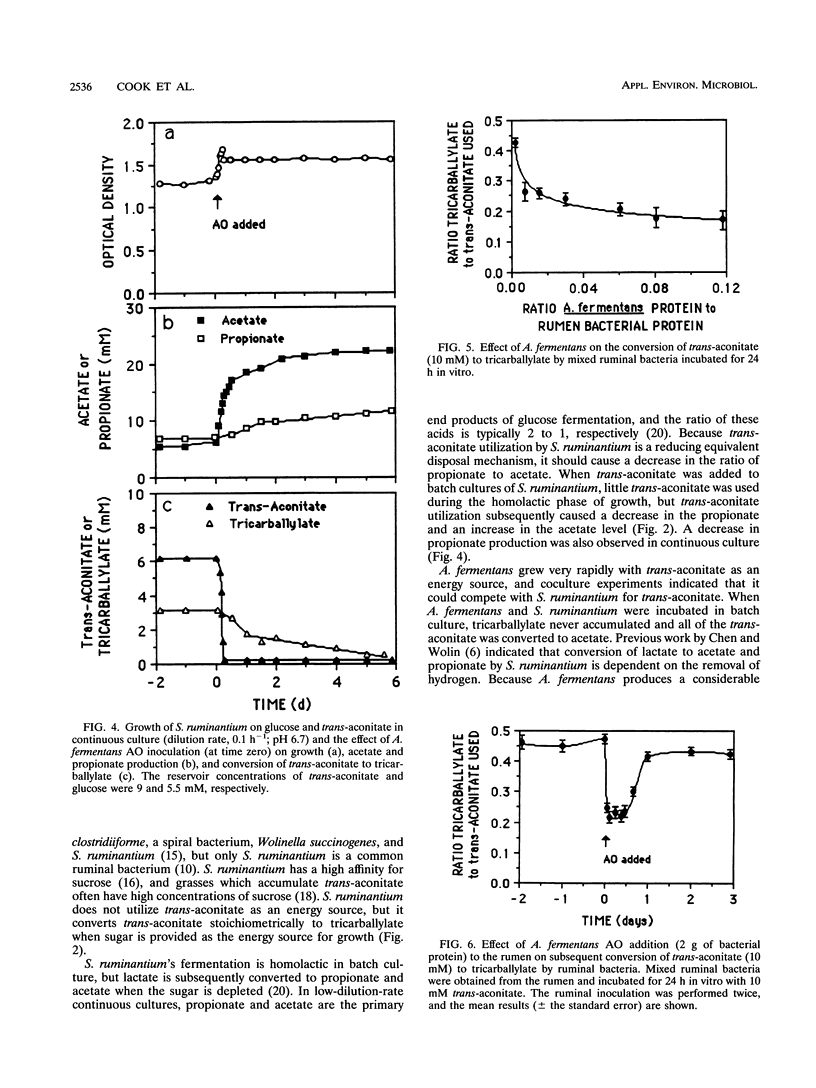
Abstract
Mixed ruminal bacteria convert trans-aconitate to tricarballylate, a tricarboxylic acid which chelates blood divalent cations and decreases their availability (J. B. Russell and P. J. Van Soest, Appl. Environ. Microbiol. 47:155-159, 1984). Decreases in blood magnesium in turn cause a potentially fatal disease known as grass tetany. trans-Aconitate was stoichiometrically reduced to tricarballylate by Selenomonas ruminantium, a common ruminal bacterium in grass-fed ruminants (J. B. Russell, Appl. Environ. Microbiol. 49:120-126, 1985). When mixed ruminal bacteria were enriched with trans-aconitate, a trans-aconitate-oxidizing bacterium was also isolated (G. M. Cook, F. A. Rainey, G. Chen, E. Stackebrandt, and J. B. Russell, Int. J. Syst. Bacteriol. 44:576-578, 1994). The trans-aconitate-oxidizing bacterium was identified as Acidaminococcus fermentans, and it converted trans-aconitate to acetate, a nontoxic end product of ruminal fermentation. When S. ruminantium and A. fermentans were cocultured with trans-aconitate and glucose, tricarballylate never accumulated and all the trans-aconitate was converted to acetate. Continuous-culture studies (dilution rate, 0.1 h-1) likewise indicated that A. fermentans could outcompete S. ruminantium for trans-aconitate. When mixed ruminal bacteria were incubated in vitro with 10 mM trans-aconitate for 24 h, 45% of the trans-aconitate was converted to tricarballylate. Tricarballylate production decreased 50% if even small amounts of A. fermentans were added to the incubation mixes (0.01 mg of protein per mg of mixed bacterial protein). When A. fermentans (2 g of bacterial protein) was added directly to the rumen, the subsequent conversion of trans-aconitate to tricarballylate decreased 50%, but this effect did not persist for more than 18 h.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohman V. R., Horn F. P., Stewart B. A., Mathers A. C., Grunes D. L. Wheat pasture poisoning. I. An evaluation of cereal pastures as related to tetany in beef cows. J Anim Sci. 1983 Dec;57(6):1352–1363. doi: 10.2527/jas1983.5761352x. [DOI] [PubMed] [Google Scholar]
- Bohman V. R., Lesperance A. L., Harding G. D., Grunes D. L. Induction of experimental tetany in cattle. J Anim Sci. 1969 Jul;29(1):99–102. doi: 10.2527/jas1969.29199x. [DOI] [PubMed] [Google Scholar]
- Burau R., Stout P. R. Trans-Aconitic Acid in Range Grasses in Early Spring. Science. 1965 Nov 5;150(3697):766–767. doi: 10.1126/science.150.3697.766. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa W. Degradation of amino acids by the mixed rumen microbial population. J Anim Sci. 1976 Oct;43(4):828–834. doi: 10.2527/jas1976.434828x. [DOI] [PubMed] [Google Scholar]
- Chen M., Wolin M. J. Influence of CH4 production by Methanobacterium ruminantium on the fermentation of glucose and lactate by Selenomonas ruminantium. Appl Environ Microbiol. 1977 Dec;34(6):756–759. doi: 10.1128/aem.34.6.756-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. M., Rainey F. A., Chen G., Stackebrandt E., Russell J. B. Emendation of the description of Acidaminococcus fermentans, a trans-aconitate- and citrate-oxidizing bacterium. Int J Syst Bacteriol. 1994 Jul;44(3):576–578. doi: 10.1099/00207713-44-3-576. [DOI] [PubMed] [Google Scholar]
- Kennedy G. S. Trans-aconitate utilization by sheep. Aust J Biol Sci. 1968 Jun;21(3):529–538. doi: 10.1071/bi9680529. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Relationship of lactate dehydrogenase specificity and growth rate to lactate metabolism by Selenomonas ruminantium. Appl Microbiol. 1975 Dec;30(6):916–921. doi: 10.1128/am.30.6.916-921.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Comparison of substrate affinities among several rumen bacteria: a possible determinant of rumen bacterial competition. Appl Environ Microbiol. 1979 Mar;37(3):531–536. doi: 10.1128/aem.37.3.531-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B. Enrichment and Isolation of Rumen Bacteria That Reduce trans- Aconitic Acid to Tricarballylic Acid. Appl Environ Microbiol. 1985 Jan;49(1):120–126. doi: 10.1128/aem.49.1.120-126.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Forsberg N. Production of tricarballylic acid by rumen microorganisms and its potential toxicity in ruminant tissue metabolism. Br J Nutr. 1986 Jul;56(1):153–162. doi: 10.1079/bjn19860095. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Van Soest P. J. In vitro ruminal fermentation of organic acids common in forage. Appl Environ Microbiol. 1984 Jan;47(1):155–159. doi: 10.1128/aem.47.1.155-159.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R., Topley M., Russell J. B. Effect of tricarballylic acid, a nonmetabolizable rumen fermentation product of trans-aconitic acid, on Mg, Ca and Zn utilization of rats. J Nutr. 1988 Feb;118(2):183–188. doi: 10.1093/jn/118.2.183. [DOI] [PubMed] [Google Scholar]



