Abstract
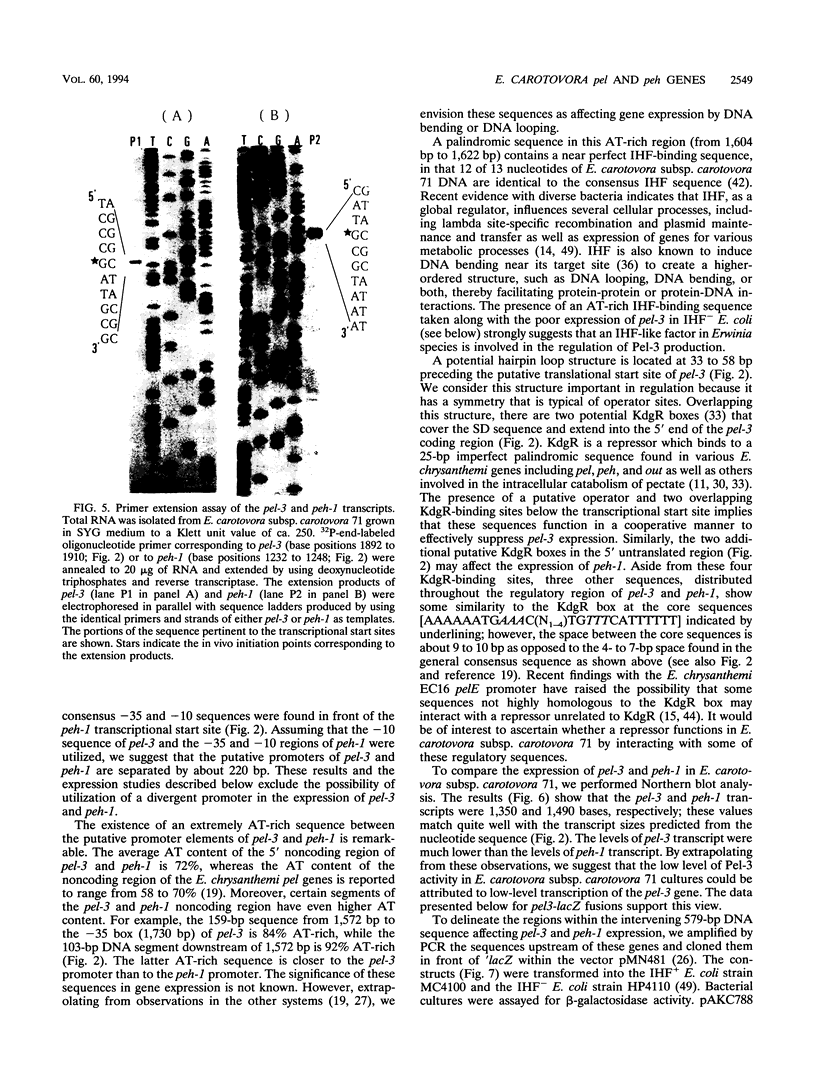
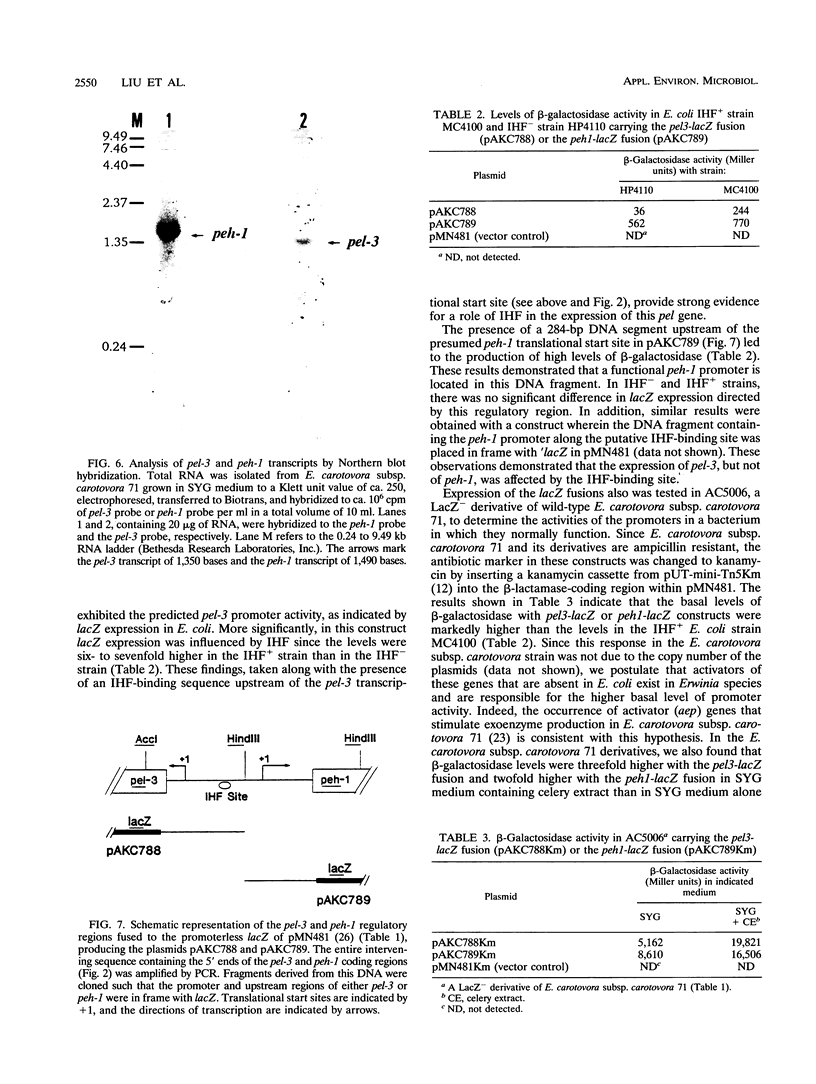
Our previous genetic analysis (J. W. Willis, J. K. Engwall, and A. K. Chatterjee, Phytopathology 77:1199-1205, 1987) had revealed a tight linkage between pel-3 (pel, pectate lyase gene) and peh-1 (peh, polygalacturonase gene) within the chromosome of Erwinia carotovora subsp. carotovora 71. Nucleotide sequencing, transcript assays, and expression of enzymatic activities in Escherichia coli have now confirmed that a 3,500-bp segment contains the open reading frames (ORFs) for Pel-3 and Peh-1. The 1,041-bp pel-3 ORF and the 1,206-bp peh-1 ORF are separated by a 579-bp sequence. The genes are transcribed divergently from their own promoters. In E. coli and E. carotovora subsp. carotovora 71, peh-1 is better expressed than pel-3. However, plant signals activate the expression of both the genes in E. carotovora subsp. carotovora. A consensus integration host factor (IHF)-binding sequence upstream of pel-3 appears physiologically significant, since pel-3 promoter activity is higher in an E. coli IHF+ strain than in an IHF- strain. While peh-1 has extensive homology with plant and bacterial peh genes, pel-3 appears not to have significant homology with the pel genes belonging to the pelBC, pelADE, or periplasmic pel families. Pel-3 also is unusual in that it is predicted to contain an ATP- and GTP-binding site motif A (P-loop) not found in the other Pels.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barras F., Thurn K. K., Chatterjee A. K. Resolution of four pectate lyase structural genes of Erwinia chrysanthemi (EC16) and characterization of the enzymes produced in Escherichia coli. Mol Gen Genet. 1987 Sep;209(2):319–325. doi: 10.1007/BF00329660. [DOI] [PubMed] [Google Scholar]
- Budelier K. A., Smith A. G., Gasser C. S. Regulation of a stylar transmitting tissue-specific gene in wild-type and transgenic tomato and tobacco. Mol Gen Genet. 1990 Nov;224(2):183–192. doi: 10.1007/BF00271551. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Buchanan G. E., Behrens M. K., Starr M. P. Synthesis and excretion of polygalacturonic acid trans-eliminase in Erwinia, Yersinia, and Klebsiella species. Can J Microbiol. 1979 Jan;25(1):94–102. doi: 10.1139/m79-014. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Thurn K. K., Tyrell D. J. Isolation and characterization of Tn5 insertion mutants of Erwinia chrysanthemi that are deficient in polygalacturonate catabolic enzymes oligogalacturonate lyase and 3-deoxy-D-glycero-2,5-hexodiulosonate dehydrogenase. J Bacteriol. 1985 May;162(2):708–714. doi: 10.1128/jb.162.2.708-714.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A., McEvoy J. L., Chambost J. P., Blasco F., Chatterjee A. K. Nucleotide sequence and molecular characterization of pnlA, the structural gene for damage-inducible pectin lyase of Erwinia carotovora subsp. carotovora 71. J Bacteriol. 1991 Mar;173(5):1765–1769. doi: 10.1128/jb.173.5.1765-1769.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvaux S., Beguin P., Aubert J. P., Bhat K. M., Gow L. A., Wood T. M., Bairoch A. Calcium-binding affinity and calcium-enhanced activity of Clostridium thermocellum endoglucanase D. Biochem J. 1990 Jan 1;265(1):261–265. doi: 10.1042/bj2650261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condemine G., Dorel C., Hugouvieux-Cotte-Pattat N., Robert-Baudouy J. Some of the out genes involved in the secretion of pectate lyases in Erwinia chrysanthemi are regulated by kdgR. Mol Microbiol. 1992 Nov;6(21):3199–3211. doi: 10.1111/j.1365-2958.1992.tb01775.x. [DOI] [PubMed] [Google Scholar]
- Friedman D. I. Integration host factor: a protein for all reasons. Cell. 1988 Nov 18;55(4):545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- Gold S., Nishio S., Tsuyumu S., Keen N. T. Analysis of the pelE promoter in Erwinia chrysanthemi EC16. Mol Plant Microbe Interact. 1992 Mar-Apr;5(2):170–178. [PubMed] [Google Scholar]
- Gysler C., Harmsen J. A., Kester H. C., Visser J., Heim J. Isolation and structure of the pectin lyase D-encoding gene from Aspergillus niger. Gene. 1990 Apr 30;89(1):101–108. doi: 10.1016/0378-1119(90)90211-9. [DOI] [PubMed] [Google Scholar]
- Hinton J. C., Gill D. R., Lalo D., Plastow G. S., Salmond G. P. Sequence of the peh gene of Erwinia carotovora: homology between Erwinia and plant enzymes. Mol Microbiol. 1990 Jun;4(6):1029–1036. doi: 10.1111/j.1365-2958.1990.tb00675.x. [DOI] [PubMed] [Google Scholar]
- Hinton J. C., Sidebotham J. M., Gill D. R., Salmond G. P. Extracellular and periplasmic isoenzymes of pectate lyase from Erwinia carotovora subspecies carotovora belong to different gene families. Mol Microbiol. 1989 Dec;3(12):1785–1795. doi: 10.1111/j.1365-2958.1989.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Hoover T. R., Santero E., Porter S., Kustu S. The integration host factor stimulates interaction of RNA polymerase with NIFA, the transcriptional activator for nitrogen fixation operons. Cell. 1990 Oct 5;63(1):11–22. doi: 10.1016/0092-8674(90)90284-l. [DOI] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N., Robert-Baudouy J. Analysis of the regulation of the pelBC genes in Erwinia chrysanthemi 3937. Mol Microbiol. 1992 Aug;6(16):2363–2376. doi: 10.1111/j.1365-2958.1992.tb01411.x. [DOI] [PubMed] [Google Scholar]
- Lei S. P., Lin H. C., Heffernan L., Wilcox G. Cloning of the pectate lyase genes from Erwinia carotovora and their expression in Escherichia coli. Gene. 1985;35(1-2):63–70. doi: 10.1016/0378-1119(85)90158-1. [DOI] [PubMed] [Google Scholar]
- Lei S. P., Lin H. C., Wang S. S., Higaki P., Wilcox G. Characterization of the Erwinia carotovora peh gene and its product polygalacturonase. Gene. 1992 Aug 1;117(1):119–124. doi: 10.1016/0378-1119(92)90499-f. [DOI] [PubMed] [Google Scholar]
- Liu Y., Murata H., Chatterjee A., Chatterjee A. K. Characterization of a novel regulatory gene aepA that controls extracellular enzyme production in the phytopathogenic bacterium Erwinia carotovora subsp. carotovora. Mol Plant Microbe Interact. 1993 May-Jun;6(3):299–308. doi: 10.1094/mpmi-6-299. [DOI] [PubMed] [Google Scholar]
- McEvoy J. L., Murata H., Chatterjee A. K. Molecular cloning and characterization of an Erwinia carotovora subsp. carotovora pectin lyase gene that responds to DNA-damaging agents. J Bacteriol. 1990 Jun;172(6):3284–3289. doi: 10.1128/jb.172.6.3284-3289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton N. P. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 1984 Nov;31(1-3):269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- Montgomery J., Pollard V., Deikman J., Fischer R. L. Positive and negative regulatory regions control the spatial distribution of polygalacturonase transcription in tomato fruit pericarp. Plant Cell. 1993 Sep;5(9):1049–1062. doi: 10.1105/tpc.5.9.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H., Fons M., Chatterjee A., Collmer A., Chatterjee A. K. Characterization of transposon insertion out- mutants of Erwinia carotovora subsp. carotovora defective in enzyme export and of a DNA segment that complements out mutations in E. carotovora subsp. carotovora, E. carotovora subsp. atroseptica, and Erwinia chrysanthemi. J Bacteriol. 1990 Jun;172(6):2970–2978. doi: 10.1128/jb.172.6.2970-2978.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser W., Reverchon S., Robert-Baudouy J. Purification and functional characterization of the KdgR protein, a major repressor of pectinolysis genes of Erwinia chrysanthemi. Mol Microbiol. 1992 Jan;6(2):257–265. doi: 10.1111/j.1365-2958.1992.tb02007.x. [DOI] [PubMed] [Google Scholar]
- Rafnar T., Griffith I. J., Kuo M. C., Bond J. F., Rogers B. L., Klapper D. G. Cloning of Amb a I (antigen E), the major allergen family of short ragweed pollen. J Biol Chem. 1991 Jan 15;266(2):1229–1236. [PubMed] [Google Scholar]
- Reverchon S., Huang Y., Bourson C., Robert-Baudouy J. Nucleotide sequences of the Erwinia chrysanthemi ogl and pelE genes negatively regulated by the kdgR gene product. Gene. 1989 Dec 21;85(1):125–134. doi: 10.1016/0378-1119(89)90472-1. [DOI] [PubMed] [Google Scholar]
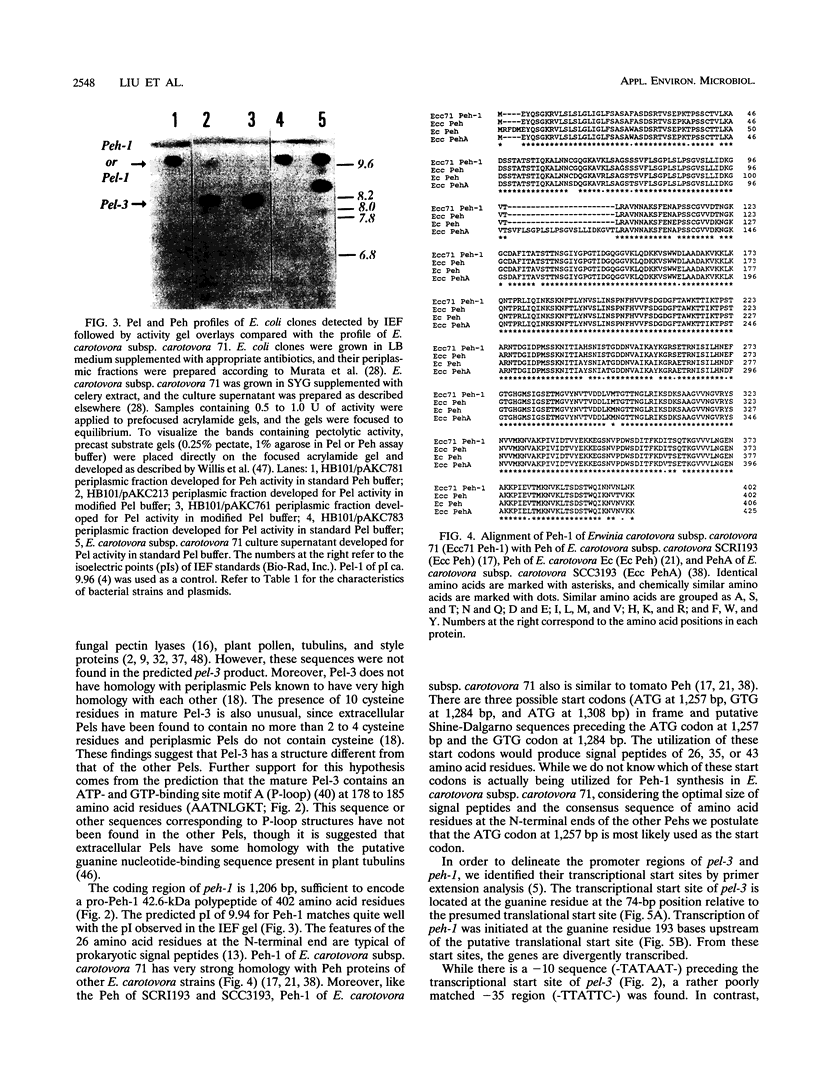
- Ried J. L., Collmer A. Activity stain for rapid characterization of pectic enzymes in isoelectric focusing and sodium dodecyl sulfate-polyacrylamide gels. Appl Environ Microbiol. 1985 Sep;50(3):615–622. doi: 10.1128/aem.50.3.615-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. P., Berman P. M., Allen C., Stromberg V. K., Lacy G. H., Mount M. S. Requirement for two or more Erwinia carotovora subsp. carotovora pectolytic gene products for maceration of potato tuber tissue by Escherichia coli. J Bacteriol. 1986 Jul;167(1):279–284. doi: 10.1128/jb.167.1.279-284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C. A., Nash H. A. Bending of the bacteriophage lambda attachment site by Escherichia coli integration host factor. J Biol Chem. 1988 Mar 15;263(8):3554–3557. [PubMed] [Google Scholar]
- Rogers H. J., Harvey A., Lonsdale D. M. Isolation and characterization of a tobacco gene with homology to pectate lyase which is specifically expressed during microsporogenesis. Plant Mol Biol. 1992 Nov;20(3):493–502. doi: 10.1007/BF00040608. [DOI] [PubMed] [Google Scholar]
- Saarilahti H. T., Heino P., Pakkanen R., Kalkkinen N., Palva I., Palva E. T. Structural analysis of the pehA gene and characterization of its protein product, endopolygalacturonase, of Erwinia carotovora subspecies carotovora. Mol Microbiol. 1990 Jun;4(6):1037–1044. doi: 10.1111/j.1365-2958.1990.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Tamaki S. J., Gold S., Robeson M., Manulis S., Keen N. T. Structure and organization of the pel genes from Erwinia chrysanthemi EC16. J Bacteriol. 1988 Aug;170(8):3468–3478. doi: 10.1128/jb.170.8.3468-3478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trollinger D., Berry S., Belser W., Keen N. T. Cloning and characterization of a pectate lyase gene from Erwinia carotovora EC153. Mol Plant Microbe Interact. 1989 Jan-Feb;2(1):17–25. doi: 10.1094/mpmi-2-017. [DOI] [PubMed] [Google Scholar]
- Wing R. A., Yamaguchi J., Larabell S. K., Ursin V. M., McCormick S. Molecular and genetic characterization of two pollen-expressed genes that have sequence similarity to pectate lyases of the plant pathogen Erwinia. Plant Mol Biol. 1990 Jan;14(1):17–28. doi: 10.1007/BF00015651. [DOI] [PubMed] [Google Scholar]
- Wu Y. F., Datta P. Integration host factor is required for positive regulation of the tdc operon of Escherichia coli. J Bacteriol. 1992 Jan;174(1):233–240. doi: 10.1128/jb.174.1.233-240.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder M. D., Keen N. T., Jurnak F. New domain motif: the structure of pectate lyase C, a secreted plant virulence factor. Science. 1993 Jun 4;260(5113):1503–1507. doi: 10.1126/science.8502994. [DOI] [PubMed] [Google Scholar]
- Zink R. T., Chatterjee A. K. Cloning and expression in Escherichia coli of pectinase genes of Erwinia carotovora subsp. carotovora. Appl Environ Microbiol. 1985 Mar;49(3):714–717. doi: 10.1128/aem.49.3.714-717.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink R. T., Engwall J. K., McEvoy J. L., Chatterjee A. K. recA is required in the induction of pectin lyase and carotovoricin in Erwinia carotovora subsp. carotovora. J Bacteriol. 1985 Oct;164(1):390–396. doi: 10.1128/jb.164.1.390-396.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Herrero M., Jakubzik U., Timmis K. N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990 Nov;172(11):6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gijsegem F. Relationship between the pel genes of the pelADE cluster in Erwinia chrysanthemi strain B374. Mol Microbiol. 1989 Oct;3(10):1415–1424. doi: 10.1111/j.1365-2958.1989.tb00124.x. [DOI] [PubMed] [Google Scholar]