Abstract
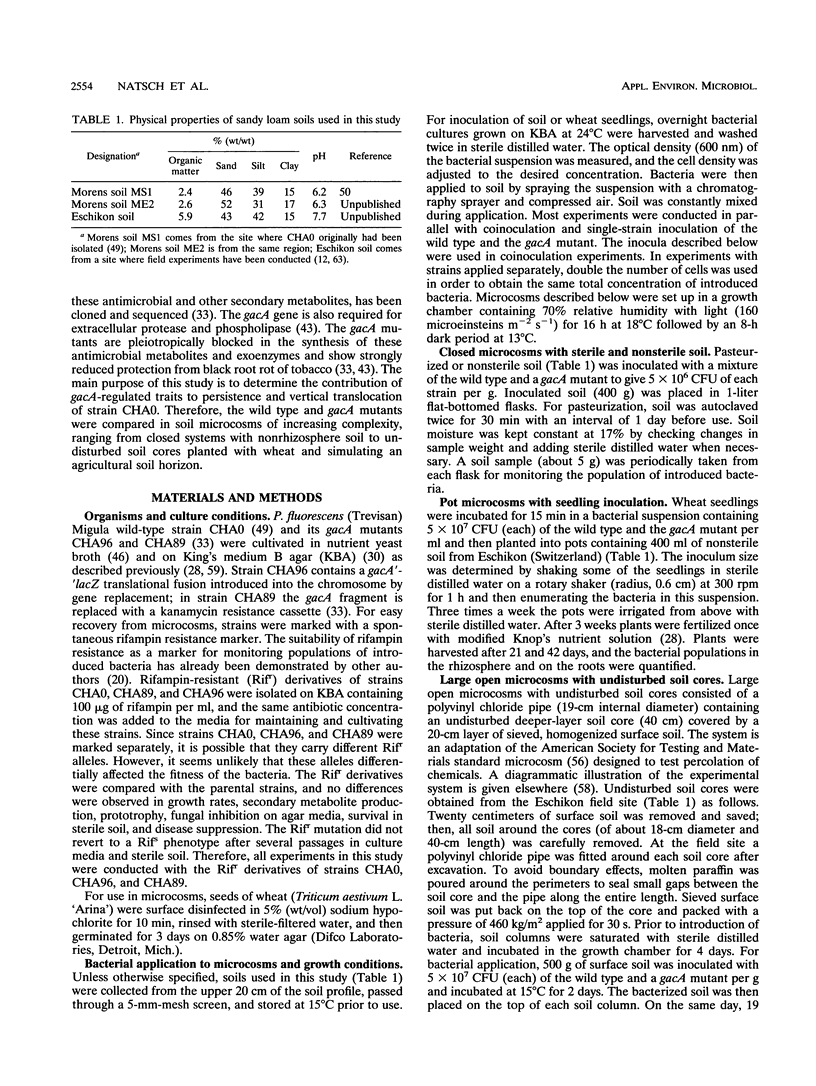
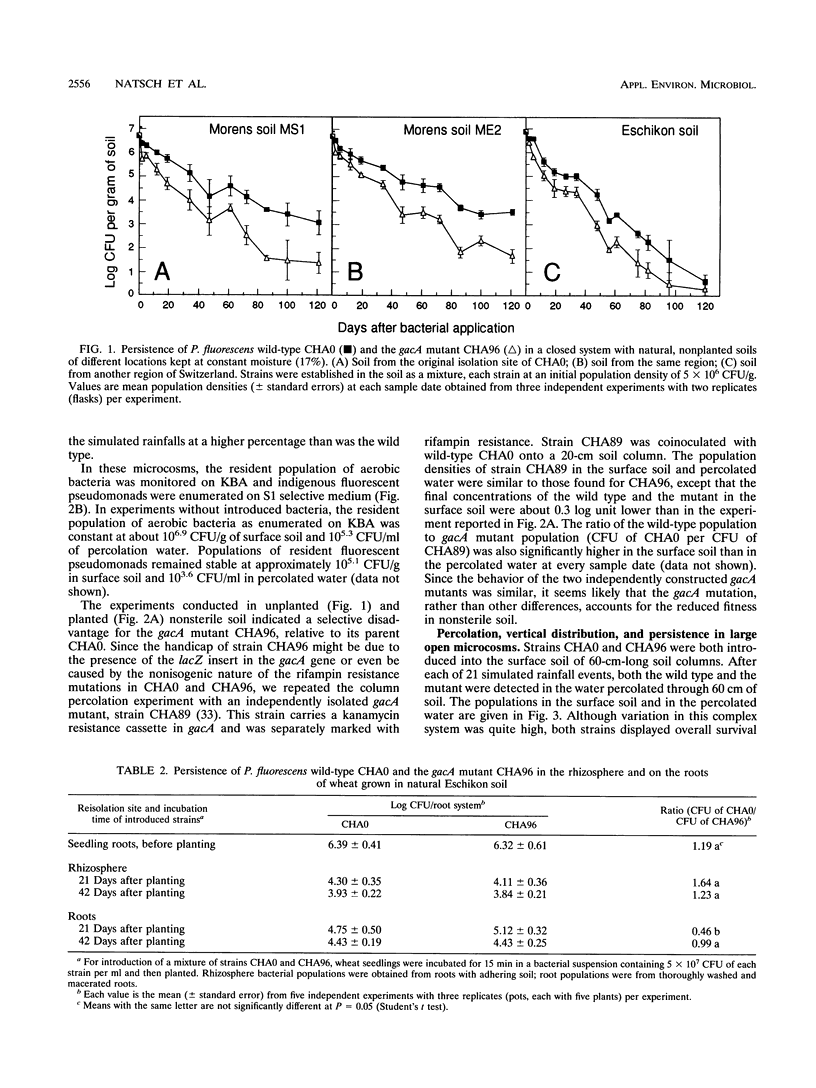
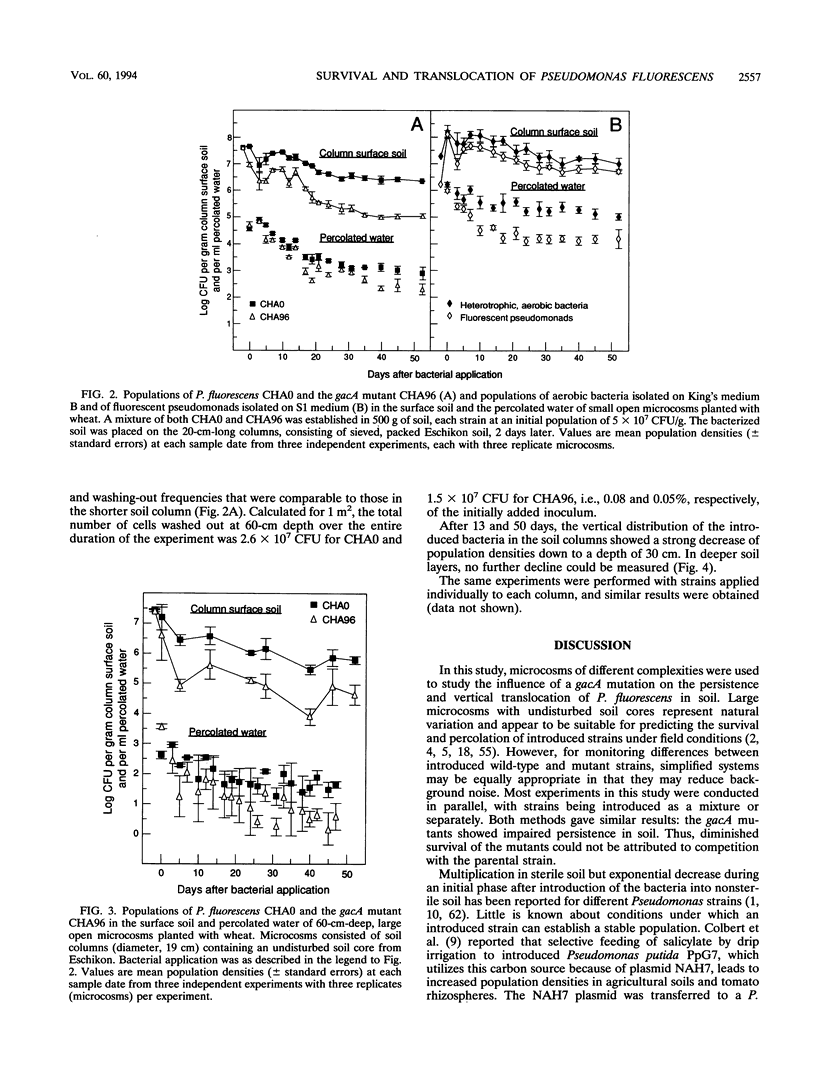
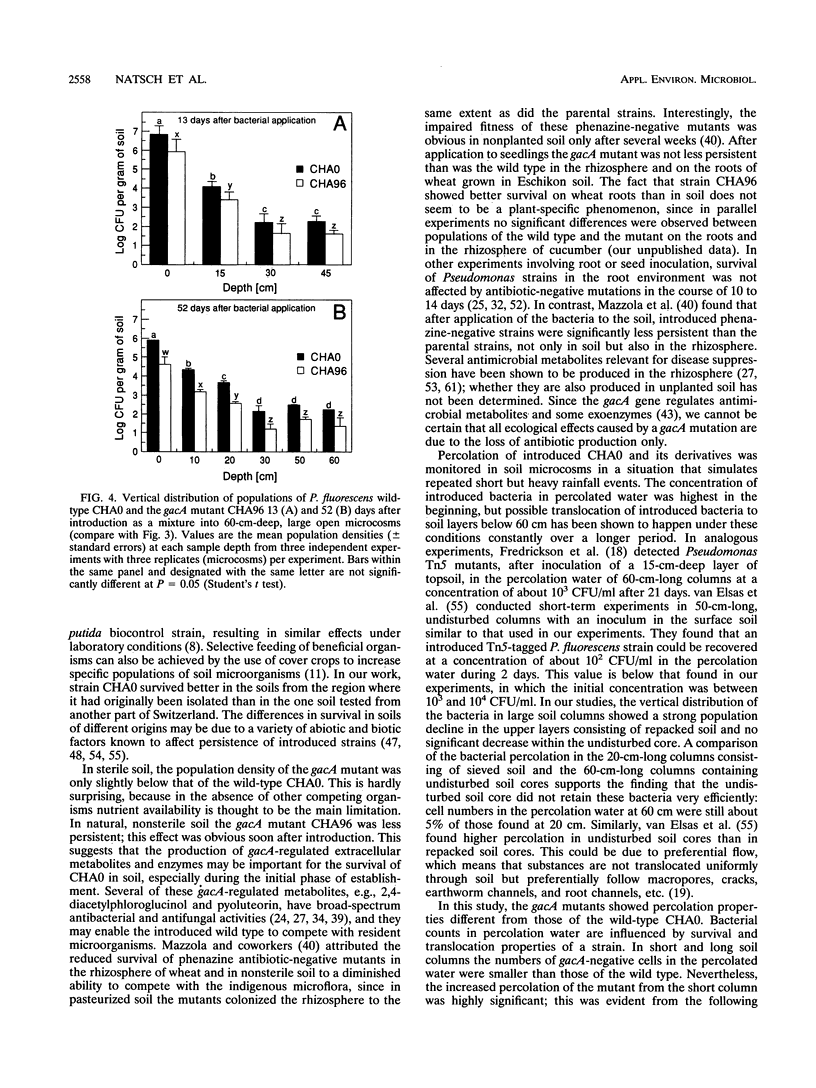
Structural and regulatory genes involved in the synthesis of antimicrobial metabolites are essential for the biocontrol activity of fluorescent pseudomonads and, in principle, amenable to genetic engineering for strain improvement. An eventual large-scale release of such bacteria raises the question of whether such genes also contribute to the persistence and dissemination of the bacteria in soil ecosystems. Pseudomonas fluorescens wild-type strain CHA0 protects plants against a variety of fungal diseases and produces several antimicrobial metabolites. The regulatory gene gacA globally controls antibiotic production and is crucial for disease suppression in CHA0. This gene also regulates the production of extracellular protease and phospholipase. The contribution of gacA to survival and vertical translocation of CHA0 in soil microcosms of increasing complexity was studied in coinoculation experiments with the wild type and a gacA mutant which lacks antibiotics and some exoenzymes. Both strains were marked with spontaneous resistance to rifampin. In a closed system with sterile soil, strain CHA0 and the gacA mutant multiplied for several weeks, whereas these strains declined exponentially in nonsterile soil of different Swiss origins. The gacA mutant was less persistent in nonrhizosphere raw soil than was the wild type, but no competitive disadvantage when colonizing the rhizosphere and roots of wheat was found in the particular soil type and during the period studied. Vertical translocation was assessed after strains had been applied to undisturbed, long (60-cm) or short (20-cm) soil columns, both planted with wheat. A smaller number of cells of the gacA mutant than of the wild type were detected in the percolated water and in different depths of the soil column. Single-strain inoculation gave similar results in all microcosms tested. We conclude that mutation in a single regulatory gene involved in antibiotic and exoenzyme synthesis can affect the survival of P. fluorescens more profoundly in unplanted soil than in the rhizosphere.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentjen S. A., Fredrickson J. K., Van Voris P., Li S. W. Intact soil-core microcosms for evaluating the fate and ecological impact of the release of genetically engineered microorganisms. Appl Environ Microbiol. 1989 Jan;55(1):198–202. doi: 10.1128/aem.55.1.198-202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert S. F., Hendson M., Ferri M., Schroth M. N. Enhanced growth and activity of a biocontrol bacterium genetically engineered to utilize salicylate. Appl Environ Microbiol. 1993 Jul;59(7):2071–2076. doi: 10.1128/aem.59.7.2071-2076.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert S. F., Schroth M. N., Weinhold A. R., Hendson M. Enhancement of Population Densities of Pseudomonas putida PpG7 in Agricultural Ecosystems by Selective Feeding with the Carbon Source Salicylate. Appl Environ Microbiol. 1993 Jul;59(7):2064–2070. doi: 10.1128/aem.59.7.2064-2070.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compeau G., Al-Achi B. J., Platsouka E., Levy S. B. Survival of rifampin-resistant mutants of Pseudomonas fluorescens and Pseudomonas putida in soil systems. Appl Environ Microbiol. 1988 Oct;54(10):2432–2438. doi: 10.1128/aem.54.10.2432-2438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deflaun M. F., Tanzer A. S., McAteer A. L., Marshall B., Levy S. B. Development of an Adhesion Assay and Characterization of an Adhesion-Deficient Mutant of Pseudomonas fluorescens. Appl Environ Microbiol. 1990 Jan;56(1):112–119. doi: 10.1128/aem.56.1.112-119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton A. M., Stephens P. M., Crowley J., O'Callaghan M., O'Gara F. Exploitation of gene(s) involved in 2,4-diacetylphloroglucinol biosynthesis to confer a new biocontrol capability to a Pseudomonas strain. Appl Environ Microbiol. 1992 Dec;58(12):3873–3878. doi: 10.1128/aem.58.12.3873-3878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould W. D., Hagedorn C., Bardinelli T. R., Zablotowicz R. M. New selective media for enumeration and recovery of fluorescent pseudomonads from various habitats. Appl Environ Microbiol. 1985 Jan;49(1):28–32. doi: 10.1128/aem.49.1.28-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Laville J., Voisard C., Keel C., Maurhofer M., Défago G., Haas D. Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1562–1566. doi: 10.1073/pnas.89.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. M., Rowley D. A., Abraham R. R. Portable infrared pupillometry using Pupilscan: relation to somatic and autonomic nerve function in diabetes mellitus. Clin Auton Res. 1992 Oct;2(5):335–341. doi: 10.1007/BF01824304. [DOI] [PubMed] [Google Scholar]
- Mazzola M., Cook R. J., Thomashow L. S., Weller D. M., Pierson L. S., 3rd Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Appl Environ Microbiol. 1992 Aug;58(8):2616–2624. doi: 10.1128/aem.58.8.2616-2624.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan D. J., O'Gara F. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev. 1992 Dec;56(4):662–676. doi: 10.1128/mr.56.4.662-676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson L. S., 3rd, Thomashow L. S. Cloning and heterologous expression of the phenazine biosynthetic locus from Pseudomonas aureofaciens 30-84. Mol Plant Microbe Interact. 1992 Jul-Aug;5(4):330–339. doi: 10.1094/mpmi-5-330. [DOI] [PubMed] [Google Scholar]
- Sacherer P., Défago G., Haas D. Extracellular protease and phospholipase C are controlled by the global regulatory gene gacA in the biocontrol strain Pseudomonas fluorescens CHA0. FEMS Microbiol Lett. 1994 Feb 15;116(2):155–160. doi: 10.1111/j.1574-6968.1994.tb06694.x. [DOI] [PubMed] [Google Scholar]
- Schroth M. N., Hancock J. G. Disease-suppressive soil and root-colonizing bacteria. Science. 1982 Jun 25;216(4553):1376–1381. doi: 10.1126/science.216.4553.1376. [DOI] [PubMed] [Google Scholar]
- Stanisich V. A., Holloway B. W. A mutant sex factor of Pseudomonas aeruginosa. Genet Res. 1972 Feb;19(1):91–108. doi: 10.1017/s0016672300014294. [DOI] [PubMed] [Google Scholar]
- Stotzky G., Zeph L. R., Devanas M. A. Factors affecting the transfer of genetic information among microorganisms in soil. Biotechnology. 1991;15:95–122. doi: 10.1016/b978-0-409-90199-3.50012-7. [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M., Bonsall R. F., Pierson L. S. Production of the antibiotic phenazine-1-carboxylic Acid by fluorescent pseudomonas species in the rhizosphere of wheat. Appl Environ Microbiol. 1990 Apr;56(4):908–912. doi: 10.1128/aem.56.4.908-912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol. 1988 Aug;170(8):3499–3508. doi: 10.1128/jb.170.8.3499-3508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M. N., Harrison L. A., Brackin J. M., Kovacevich P. A., Mukerji P., Weller D. M., Pierson E. A. Genetic analysis of the antifungal activity of a soilborne Pseudomonas aureofaciens strain. Appl Environ Microbiol. 1991 Oct;57(10):2928–2934. doi: 10.1128/aem.57.10.2928-2934.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisard C., Keel C., Haas D., Dèfago G. Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J. 1989 Feb;8(2):351–358. doi: 10.1002/j.1460-2075.1989.tb03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



