Abstract
The left atrium (LA) anterior–posterior diameter was one of the first standardised echocardiographic parameters. However, the clinical importance of LA size assessment has been neglected for some time. Recent population‐based studies have demonstrated the prognostic value of LA size for long‐term outcome. Furthermore, with new dedicated techniques such as tissue Doppler imaging, it has become feasible to assess (regional) LA function. In addition, the introduction of catheter ablation procedures has changed the treatment of patients with drug‐refractory atrial fibrillation (AF) dramatically. New image integration systems have become available for these catheter ablation procedures. With the use of image integration systems, a real anatomical “roadmap” of the LA is provided for catheter ablation procedures. All these factors may explain the renewed interest in LA anatomy.
In this article, the importance of assessment of LA size and LA anatomy is discussed. Furthermore, the various imaging modalities that are available for the non‐invasive visualisation of the LA will be reviewed. In addition, the role of these imaging techniques in catheter ablation procedures for AF will be discussed.
Causes and mechanisms of LA dilatation
In large population‐based studies, it has been demonstrated that LA size is an important predictor of cardiovascular outcome.1,2,3 Tsang et al.3 recently demonstrated that a larger indexed LA volume predicted a higher risk of cardiovascular events after adjustment for age, gender and other covariates. Patients with a severely increased left atrium (⩾40 ml/m2) had the highest risk for the development of cardiovascular events (hazard ratio 6.6).3
Left atrium dilatation can occur in a broad spectrum of cardiovascular diseases including hypertension, left ventricular dysfunction, mitral valve disease and AF. In general, two major conditions are associated with LA dilatation: pressure overload and volume overload.4 LA volume overload frequently occurs in the setting of mitral regurgitation. Pressure overload is most frequently caused by an increased LA afterload, secondary to mitral valve disease or left ventricular dysfunction.4 Pritchett et al.5 demonstrated a close correlation between LA volume and the severity of diastolic dysfunction after adjusting for the presence of covariates including age, gender, cardiovascular disease, ejection fraction and left ventricular mass. Accordingly, it has been suggested that whereas LA volumes represent long‐term exposure to elevated pressures, Doppler measures of filling pressures rather represent the actual left ventricular filling pressures at one point in time.6
Atrial fibrillation is another important factor associated with LA dilatation. Atrial fibrillation is the most commonly encountered cardiac arrhythmia, and the association of LA enlargement and AF has been well recognised.1,7,8,9,10 However, whether AF causes LA dilatation or vice versa still remains controversial. Several studies suggest that LA enlargement may cause AF.1,7,8 In the Framingham Heart Study,7 M‐mode derived LA size was an independent risk factor for development of AF. More recently, Tsang et al.1 demonstrated that LA volume (assessed with a modified biplane method) was a strong predictor of AF, incremental to clinical risk factors.1 However, other studies have revealed that LA enlargement may be the consequence of AF.9,10 Dittrich et al.10 demonstrated that AF was an independent predictor of LA size in a large cohort study with 3465 patients with AF.
The importance of LA size/anatomy assessment
Assessment of LA size is important since it has been shown to provide strong prognostic information. The incremental value of LA size over conventional risk factors has been demonstrated in several studies.3,11,12,13 In the Framingham Heart study13 it was demonstrated that LA enlargement was a significant predictor of death in both men and women. The relative risk of death per 10 mm increment in LA size was 1.3 for men (95% CI 1.0 to 1.5) and 1.4 for women (95% CI 1.1 to 1.7).
In particular, assessment of LA size is important in patients with AF. The guidelines on management of patients with AF recommend a standard two‐dimensional and Doppler echocardiogram, with assessment of LA size and function, in the clinical evaluation of all patients with AF.14 Osranek et al.12 demonstrated the predictive value of LA dilatation in patients with lone AF. In this population‐based study with a median follow‐up of 27 years, it was noted that in patients with lone AF, LA volume was a strong predictor of adverse events (cerebrovascular event, acute myocardial infarction, heart failure, hospitalization, death), independent of age and clinical risk factors.12
The assessment of LA anatomy is important in the setting of catheter ablation procedures for AF. Although there is still debate concerning the best ablation strategy and the exact lesion set, knowledge on LA and pulmonary vein anatomy is mandatory, both before and during the ablation procedure. Anatomical15 and in vivo studies with different imaging modalities16,17,18 have shown that LA and pulmonary vein anatomy is highly variable.
Different non‐invasive imaging modalities are available for assessment of LA size and anatomy. The various techniques and their clinical relevance/applications will be discussed in the following paragraphs.
Multi‐modality imaging of the LA
Echocardiography
For assessment of LA size various echocardiographic techniques are available, including transthoracic, transoesophageal and intracardiac echocardiography. Transthoracic echocardiography is most commonly used in daily clinical practice to assess LA size. Transoesophageal and intracardiac echocardiography are mainly used during interventions for AF, such as cardioversion (transoesophageal echocardiography) and catheter ablation procedures (intracardiac echocardiography).
Transthoracic echocardiography
Feigenbaum was the first to demonstrate the correlation between LA dimension assessed with M‐mode echocardiography and angiographic LA size.19 Afterwards, the development of two‐dimensional echocardiography has expanded the insight into LA size and morphology. Nowadays, various established parameters for assessment of LA size are available.20 The LA anteroposterior diameter as assessed with M‐mode is most commonly used in daily clinical practice and in large studies. However, it is not sufficient to determine true LA size, since M‐mode represents only one dimension of the LA.21 In particular in LA enlargement, which may result in an asymmetrical geometry of the LA, M‐mode echocardiography may underestimate LA size. Therefore, optimal assessment of LA size should include LA volume measurements.20,21 Various methods for the assessment of LA volume with two‐dimensional echocardiography are available, including the cubical method, area–length method, ellipsoid method and modified Simpson's rule (table 1, fig 1). In a prospective study including 631 patients,22 it was demonstrated that the biplane area–length method and the biplane Simpson's method compared closely (mean LA volume 39±14 ml/m2 and 38±13 ml/m2, correlation coefficient 0.98), whereas the ellipsoid method systematically underestimated LA volume (mean LA volume 32±14 ml/m2).
Table 1 Methods for left atrial volume quantification with two‐dimensional echocardiography.
| Method | Parameter | View | Equation | Assumption |
|---|---|---|---|---|
| Cube | APD | PSLAX | 4/3π (APD/2)3 | LA has a spherical shape |
| Ellipsoid | APD | PSLAX | 4/3π (APD/2)(D1/2)(L/2) | LA has an ellipsoid geometry |
| LA transversal diameter (D1) | 4CH | |||
| LA length (L) | 4CH | |||
| Area–length | LA area planimetry (A2C) | 2CH | 8/3π [(A4C)(A2C)/L] | LA has an ellipsoid geometry |
| LA area planimetry (A4C) | 4CH | |||
| LA length (L) | 2CH or 4CH | |||
| Modified Simpson's rule | LA planimetry | 2CH | Summation of discs | Total volume can be calculated from sum of smaller volumes |
| 4CH |
APD, anterior–posterior diameter; LA, left atrium; PSLAX, parasternal long‐axis; 2CH, two‐chamber view; 4CH, four‐chamber view.
Figure 1 Measurement of LA volumes with transthoracic echocardiography using the modified biplane Simpson's rule. The maximum LA volume is assessed during ventricular systole in the apical two‐chamber (left panel) and apical four‐chamber (right panel) views. Maximal LA volume was 46 ml, minimal LA volume was 22 ml, resulting in an LA ejection fraction of 53%.
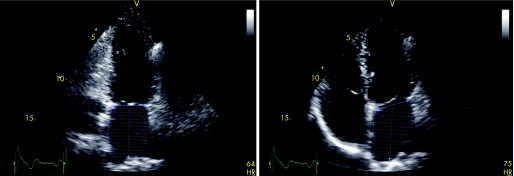
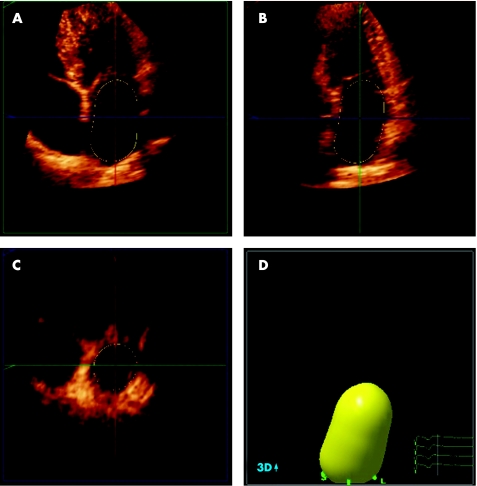
Recently, three‐dimensional echocardiography has been introduced (fig 2). A number of studies have demonstrated the feasibility of three‐dimensional echocardiography for the assessment of LA volumes,23,24 and it has been validated against MRI.25 Jenkins et al.23 have demonstrated that three‐dimensional echocardiography allows accurate LA volume assessment, with a low test–retest variation, and a lower intraobserver and interobserver variability as compared to two‐dimensional echocardiography. However, there still remain some technical limitations such as the spatial and temporal resolution. In addition, since a relatively constant R–R interval is needed, three‐dimensional echocardiography may not be feasible in patients with AF and a high ventricular response rate.
Figure 2 Real‐time three‐dimensional echocardiogram for the assessment of LA volumes. Panels A to C represent the coronal, sagittal and transverse planes, respectively. With the use of a five‐point tracing algorithm, LA volumes can be obtained throughout the cardiac cycle, represented by the “shell” in panel D. In this example, LA maximum volume was 53 ml.
Transoesophageal echocardiography
Transoesophageal echocardiography provides good views on the LA and the LA appendage. However, visualising the complete left atrium to determine LA size with transoesophageal echocardiography may be hampered by the close proximity of the probe to the LA and the variable position of the oesophagus to the posterior LA. As a result, measurements of LA size with transoesophageal echocardiography have not been standardised. Few studies have compared the assessment of LA size with transoesophageal echocardiography and transthoracic echocardiography. Block et al.26 assessed different LA dimensions with transoesophageal echocardiography and transthoracic echocardiography in 109 patients. The authors noted that the 30‐degree to 60‐degree short‐axis equivalent at the level of the aortic valve was the only view in which the entire LA dimension could be reliably obtained. Although transoesophageal echocardiography slightly underestimated LA size, it provided good correlation with transthoracic echocardiography.26
Transoesophageal echocardiography is considered the procedure of choice for assessment of thrombi in the LA cavity or LA appendage. It can detect thrombi with a high degree of sensitivity and specificity varying from 93% to 100%.27 In addition, transoesophageal echocardiography is helpful in assessment of LA appendage emptying velocities, which are correlated with thrombus formation (velocities <20 cm/s) and with maintenance of sinus rhythm after cardioversion (velocities >40 cm/s).28 Furthermore, transoesophageal echocardiography may be of great value in performing trans‐septal punctures in AF ablation procedures.
Intracardiac echocardiography
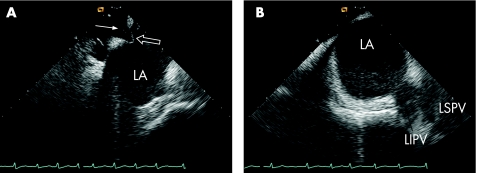
Intracardiac echocardiography (ICE) is only used during interventional procedures, such as percutaneous closure of atrial septal defects and catheter ablation procedures. Therefore, no standardised measurements of LA size or volume are available. During these interventional procedures, ICE can accurately visualise LA anatomy and related structures.29 Furthermore, it allows visualisation of intracardiac devices and catheters, and is helpful in monitoring potential complications during catheter ablation procedures.30 Examples of intracardiac echocardiograms are shown in fig 3.
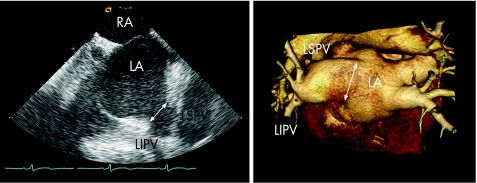
Figure 3 Intracardiac echocardiography (ICE) during a catheter ablation procedure for AF. (A) The trans‐septal puncture is guided by ICE to gain access to the LA. The white arrow indicates the trans‐septal sheath aimed at the fossa ovalis, which is the preferred site for the trans‐septal puncture. The “tenting” of the septum (open arrow) indicates stable contact of the sheath with the fossa ovalis. (B) The anatomy of the pulmonary veins can be assessed with ICE during the ablation procedure. In this patient, the left inferior pulmonary vein (LIPV) and the left superior pulmonary vein (LSPV) have a single insertion in the LA, a so‐called “common ostium” of the left‐sided pulmonary veins.
In addition, the Doppler capacities of ICE allow for monitoring of pulmonary vein narrowing and may predict the recurrence of AF after ablation.31 Furthermore, LA function can be assessed with ICE. Rotter et al.32 demonstrated a good correlation between ICE and transoesophageal echocardiography for measurement of mitral E wave velocity (correlation coefficient 0.759, mean difference 6.9 cm/s) and LA appendage emptying velocity (correlation coefficient 0.991, mean difference 0.7 cm/s). Although ICE is limited by the monoplane character and the lack of standardised measurements of LA size, it is a valuable tool for interventional procedures.
Multi‐slice CT
The application of multi‐slice CT (MSCT) in cardiac imaging has rapidly expanded in the past few years. Since MSCT has an excellent spatial and temporal resolution, it can accurately quantify LA volumes, by using the modified Simpson's method.33 However, because of the radiation exposure and the use of contrast agents, MSCT is not routinely used for the assessment of LA size.
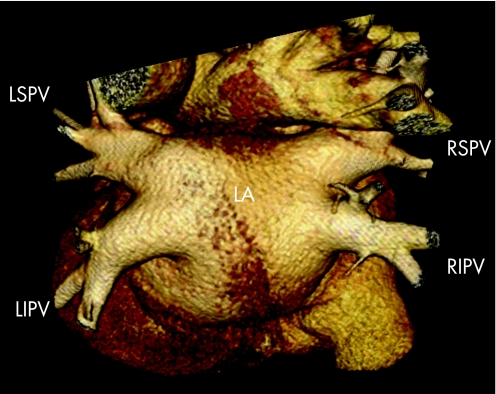
For AF ablation procedures, MSCT is a valuable tool to depict LA anatomy.34 With the use of volume‐rendered reconstructions, MSCT can provide detailed information on LA and pulmonary vein anatomy (fig 4). Since LA and pulmonary vein anatomy is highly variable, MSCT may offer a “roadmap” for ablation. The exact role of MSCT in ablation procedures is discussed later.
Figure 4 Volume‐rendered three‐dimensional reconstruction of a 64‐slice MSCT scan. The dorsal view clearly demonstrates the anatomy of the LA and pulmonary veins. In this patient, normal pulmonary vein anatomy is present including four pulmonary veins, all with their own insertion into the LA. LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is considered the most accurate technique for the non‐invasive assessment of atrial volumes, because of the high spatial resolution and the excellent myocardial border detection. Detailed information of LA size and volumes throughout the cardiac cycle can be acquired with MRI (fig 5). Anderson et al.35 recently reported findings on LA dimensions and LA area assessed with MRI in 20 healthy controls and in 20 patients with cardiomyopathy. It was noted that a LA systolic area <24 cm2 was the upper 95th percentile of the normal range, and best discriminated normal from abnormal hearts.35 Similar to MSCT, a modified Simpson's method can be used to determine LA volumes. However, due to its relatively long acquisition times and the cumbersome data analysis, LA volume assessment with MRI is not performed in daily clinical practice. MRI can provide detailed information on LA and pulmonary vein anatomy before catheter ablation procedures, and is a useful tool in the follow‐up of patients after the ablation procedure. This will be discussed in more depth later.
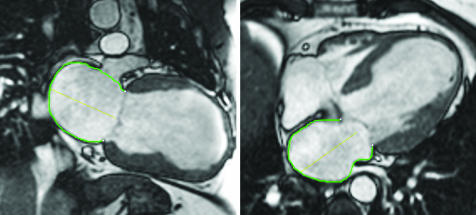
Figure 5 Assessment of LA volumes with MRI. The two‐chamber (left panel) and the four‐chamber (right panel) views are used to delineate the endocardial border of the LA (green line), as well as the maximal diameter (yellow line).
Several studies have compared the value of the different imaging modalities for the assessment of LA size and volumes.23,24,25,26,36 Two‐dimensional transthoracic echocardiography (using the biplane methods) may underestimate true LA size, as compared with CT36 or MRI.25 However, these three‐dimensional techniques are not preferred for LA size assessment in daily clinical practice. In this respect, new three‐dimensional echocardiography is a promising technique that is widely available and provides accurate information on LA size.24
Assessment of regional LA function
Regional LA function is not routinely assessed, and therefore no standardised parameters for regional LA function are available. This can be partly explained by the fact that non‐invasive evaluation of regional LA function may be hampered by the relatively thin LA walls. However, assessment of regional LA function may provide more insight in atrial electromechanical remodelling and may be helpful in the management of AF with surgical or catheter ablation. New echocardiographic techniques, such as tissue Doppler imaging and strain (rate) imaging, allow non‐invasive measurement of regional function of the myocardium. Tissue Doppler imaging quantifies regional tissue velocities of the myocardium. Strain and strain rate represent local tissue deformation and the rate (speed) of local deformation, respectively.37 Both techniques have been well validated for the assessment of regional left ventricular function. Recently, several studies have applied these new techniques to the LA.38,39,40,41,42
Tissue Doppler imaging allows quantification of regional myocardial velocities, and assessment of the timing of peak systolic and diastolic velocities of the myocardium (fig 6A). Thomas et al.38 used tissue Doppler imaging in 92 healthy volunteers to evaluate regional LA function. The authors noted that atrial contraction velocities were significantly increased in the annular segments, compared with the more superior segments.
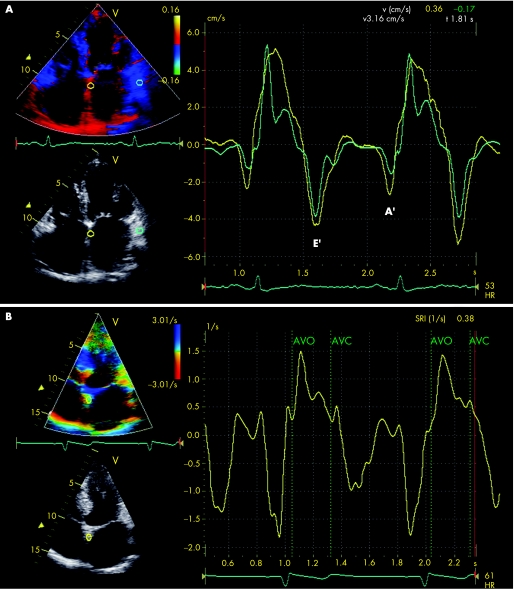
Figure 6 (A) Colour‐coded tissue Doppler imaging in the apical four‐chamber view for the assessment of regional LA function. The samples are placed in the basal atrial septum (yellow curve) and the basal atrial lateral wall (green curve). From the myocardial velocity curves, peak systolic and diastolic velocities can be assessed. Early diastolic filling is indicated by E' and late diastolic filling is indicated by A'. (B) Strain rate imaging in the apical four‐chamber view in a patient with a history of paroxysmal atrial fibrillation. A sample is placed in the basal atrial septum. From the time–strain curves segmental atrial contraction and time‐to‐peak strain can be derived. The vertical green lines indicate aortic valve opening (AVO) and aortic valve closure (AVC).
Tissue Doppler imaging also provides information on the timing of regional velocities of the myocardium. Therefore, it may quantify regional electromechanical LA function, such as the total electromechanical activity of the atria (represented by the interval between the onset of the P‐wave on the ECG to the end of the A' wave on the tissue Doppler images).39 However, the clinical relevance and the exact correlation of these new tissue Doppler‐derived parameters of regional LA function with conventional parameters, such as mitral inflow A wave velocity and LA volumes, needs further investigation. Furthermore, a limitation of tissue Doppler imaging for evaluation of regional LA function is the angle dependency of the technique. Therefore, careful adjustment of the beam and gain settings should be made to avoid aliasing and to allow reliable measurement of tissue velocities of the LA.
Strain imaging and strain rate imaging are new tools for the assessment of regional myocardial deformation of the LA.40 An example of strain rate imaging of the LA is shown in fig 6B. In contrast to tissue Doppler imaging, strain imaging is not hampered by myocardial tethering. Furthermore, strain imaging allows for differentiation between active contraction and passive motion.37 However, the thin atrial walls may not generate clear strain curves and therefore require careful interpretation. Several studies have demonstrated the value of regional atrial strain in the analysis of patients with AF undergoing cardioversion.41,42 Di Salvo et al.41 studied 65 patients with AF and performed tissue Doppler imaging of standard apical images of the LA. It was noted that all tissue Doppler imaging‐derived parameters of the LA, including tissue velocities, strain and strain rate, were significantly reduced in patients with AF, compared with healthy controls. Of interest, multivariable analysis demonstrated that atrial inferior wall peak systolic strain rate and atrial septal peak systolic strain were the best predictors of maintenance of sinus rhythm after cardioversion.41 The assessment of regional LA function by tissue Doppler imaging or strain imaging may be of value in the clinical follow‐up of patients with AF undergoing catheter ablation or cardioversion. It has been suggested that diminished regional atrial strain values may warrant prolonged use of anti‐arrhythmic drugs and anticoagulation.41,42 However, more studies are needed to appreciate the value of regional LA strain and its role to guide use of medication in patients with AF.
Imaging of LA function in therapy for AF
As previously discussed, the association between LA remodelling and AF has been well recognised. Restoration of normal sinus rhythm by catheter ablation or cardioversion may result in reverse remodelling of the LA, with subsequent improvement of LA function. However, electrical or pharmacological cardioversion may cause transient atrial mechanical dysfunction or “stunning”.28,43 It has been demonstrated that conventional parameters of LA function, such as A wave velocity or A wave velocity time integral, are decreased immediately after cardioversion.43 The subsequent depressed LA appendage flow velocities increase the risk of thromboembolic events after cardioversion.
With the use of new techniques such as strain imaging, LA dysfunction following cardioversion can also be assessed.42 In 37 patients with chronic AF, it was noted that immediately after cardioversion regional LA function was depressed compared with healthy controls. However, 6 months after successful cardioversion a significant increase in LA strain was observed. The maximal increase in regional LA strain occurred within 1 month after cardioversion.42 This observation is in concordance with previous studies43,44 and suggests that “atrial stunning” following cardioversion is a function of the preceding AF, rather than the cardioversion itself.
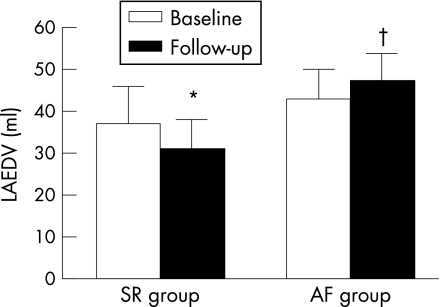
Catheter ablation has been demonstrated to be successful in the restoration of sinus rhythm, and is performed in an increasing number of patients with symptomatic drug‐refractory AF. It has been demonstrated that maintenance of sinus rhythm after catheter ablation is associated with a decrease in LA volumes (fig 7).45 Reant et al. studied 48 patients with lone AF treated with catheter ablation.46 Serial echocardiograms up to 12 months after the procedure revealed a progressive decrease in LA dimensions. Interestingly, with the use of new tissue Doppler‐derived parameters, it was noted that in parallel with the improvement in LA function, both LV systolic and diastolic function improved in the patients who maintained sinus rhythm.46 Furthermore, with the use of MRI it has been demonstrated that in addition to LA reverse remodelling, the area of the pulmonary venous ostia may decrease after successful catheter ablation procedures.47
Figure 7 Reverse remodelling of the LA may occur after successful catheter ablation. A significant reduction in LA end‐diastolic volume (LAEDV) was observed in the patients who maintained sinus rhythm after the catheter ablation procedure (SR group). In contrast, an increase in LA volume was observed in the patients who had recurrence of atrial fibrillation (AF group). *p<0.01 baseline vs 3 months of follow‐up; †p<0.05 SR group vs AF group.
Imaging in catheter ablation procedures for AF
Multi‐modality imaging
Catheter ablation procedures are being performed in an increasing number of patients worldwide. The recent guidelines on management of patients with AF propose catheter ablation as a reasonable option when first‐line anti‐arrhythmic drugs have failed.14 Various ablation strategies have been proposed, including segmental ostial ablation and anatomically based circumferential ablation, and there is still debate concerning the exact lesion set. Regardless of the ablation strategy applied, knowledge of the complex LA and pulmonary vein anatomy is essential during the ablation procedure. The venoatrial junctions and anatomical landmarks in the LA, such as the ridge between the left superior pulmonary vein and the LA appendage, are critical structures to identify during catheter ablation procedures.
Anatomical studies have demonstrated that LA and pulmonary vein anatomy is highly variable.15 Most frequently, two left‐sided pulmonary veins and two right‐sided pulmonary veins drain separately into the LA (fig 4). Anatomical variations include a single insertion or “common ostium” of the pulmonary veins, and an additional pulmonary vein (fig 8). A common ostium is most frequently found on the left‐sided pulmonary veins, whereas an additional pulmonary vein is most frequently noted on the right side. In 201 patients undergoing MSCT scanning, Marom et al.48 noted a left‐sided common ostium in 14% of the patients, and an additional right‐sided pulmonary vein in 28% of the patients. In addition, variations in LA appendage morphology and LA roof anatomy may be present in patients with AF.49 Because of the complex anatomy of the LA and the variability in pulmonary vein anatomy, a detailed roadmap for the ablation procedure is mandatory. The various imaging modalities that are available for assessment of LA and pulmonary vein anatomy in catheter ablation procedures include MSCT, MRI, ICE and electroanatomical mapping systems.
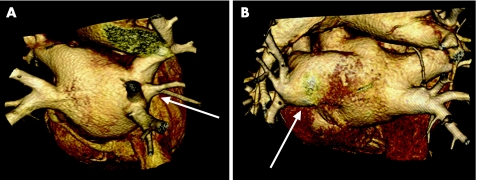
Figure 8 Variations in pulmonary vein (PV) anatomy shown on volume‐rendered three‐dimensional reconstructions of MSCT scans. (A) There is an additional PV on the right side. (B) A “common ostium” of the left‐sided PVs.
MSCT and MRI (figs 8 and 9) provide detailed information on the anatomy of the LA and pulmonary veins. With the use of volume‐rendered three‐dimensional reconstructions and cross‐sectional images, the number of pulmonary veins and their branching pattern can be accurately assessed (fig 10). Furthermore, the diameters of the pulmonary vein ostia can be measured on the different orthogonal planes. In addition, MSCT may identify the presence of thrombi in the LA appendage and provide detailed information on surrounding structures, such as the oesophagus and coronary arteries. However, the pre‐procedural acquired MSCT and MRI images only provide offline information.
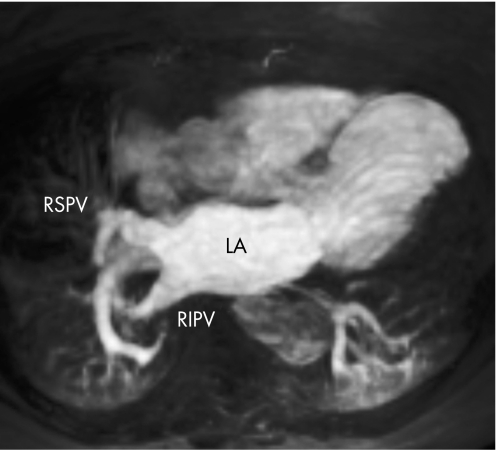
Figure 9 Maximum intensity projection of a gadolinium contrast‐enhanced MRI angiogram of the pulmonary veins. LA, left atrium; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
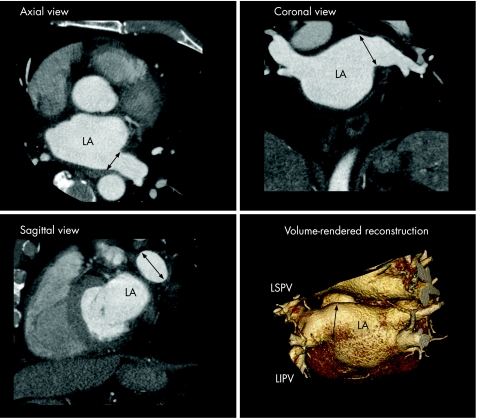
Figure 10 The different orthogonal planes (axial, coronal and sagittal) and three‐dimensional volume‐rendered reconstructions of the MSCT scan can be used to assess LA and pulmonary vein anatomy. The black arrows indicate the large “common ostium” of the left‐sided pulmonary veins. LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein.
In contrast to MSCT and MRI, ICE allows real‐time assessment of the pulmonary veins and the venoatrial junction during the ablation procedure. The Doppler capacities and the ability to monitor the catheter position in relation to the pulmonary veins are great advantages of this technique.29 In addition, ICE is helpful in assessment of the transmural extent of the ablation lesions50 and ICE has been used to titrate ablation energy, thereby increasing the safety of the ablation procedure.30 Still, the major limitation of ICE is the two‐dimensional character of the technique. It has been demonstrated that three‐dimensional imaging modalities provide the most accurate information on LA and pulmonary vein anatomy.16,17 In a direct comparison between MSCT and ICE (fig 11), it was noted that MSCT has a higher sensitivity for the detection of additional pulmonary veins and that ICE underestimated the size of the pulmonary venous ostia.16
Figure 11 Comparison of MSCT and ICE to evaluate LA and pulmonary vein anatomy. In this patient, both techniques clearly visualise the “common ostium” of the left‐sided pulmonary veins (indicated with the white arrow). Whereas MSCT may provide more detailed three‐dimensional information, ICE also provides online information on the relationship between the ablation catheter and the pulmonary veins during the actual ablation. LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RA, right atrium.
During the ablation procedure, electroanatomical mapping systems such as Carto (Biosense‐Webster, Diamond Bar, California, USA) are available to assess LA and pulmonary vein anatomy. These systems combine online electrophysiological data with anatomical information, acquired with mapping catheters positioned in the LA.51 When performing the actual ablation, the ablation points can be marked on the acquired electroanatomical map. The major limitation of these systems is the use of reconstructed anatomy. Ideally, the detailed anatomical information acquired with three‐dimensional imaging techniques (such as MSCT and MRI) could be combined with the online electrophysiological information. Recently, image integration systems have been introduced that allow the online use of MSCT or MRI images during the actual ablation procedures.
Image integration
With the use of new image integration systems such as CartoMerge (Biosense‐Webster, Diamond Bar, California, USA), it has become feasible to merge pre‐procedural acquired three‐dimensional MSCT or MRI images with online acquired electroanatomical maps. Before the ablation procedure, the MSCT or MRI images are segmented into different structures (fig 12). During the ablation procedure, the MSCT or MRI images are “registered” with the use of dedicated software algorithms that minimise the distance between the electroanatomical mapping points and the three‐dimensional MSCT or MRI images (fig 13). Both pre‐clinical52 and clinical studies53,54,55 have demonstrated the accuracy of image integration systems. Advantages of the image integration systems include the possibility to monitor the exact catheter position in relation to the endocardial border, the pulmonary veins and the surrounding structures (fig 14). Hereby, potential complications such as pulmonary vein stenosis and atrio‐oesophageal fistula may be avoided.
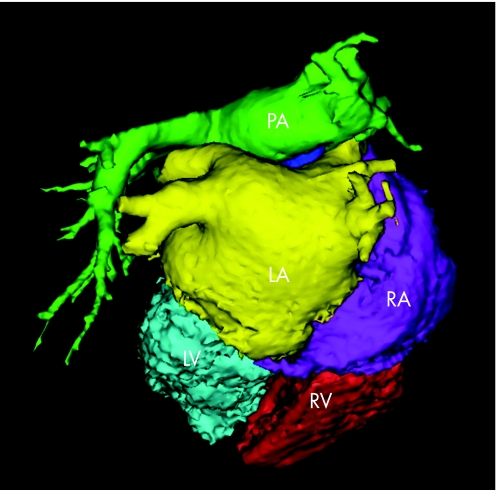
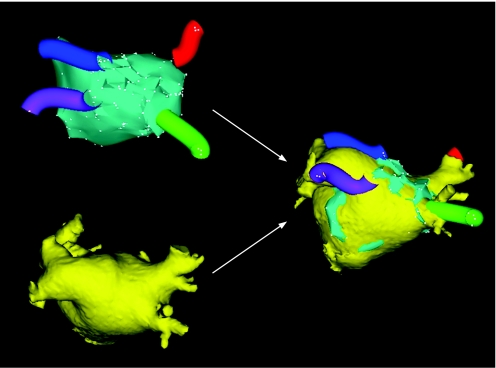
Figure 12 Segmentation of the MSCT scan into the different structures using the CartoMerge Image Integration Module. From the raw MSCT data, a three‐dimensional volume is created. This volume is divided in the different chambers by placing anatomical landmarks and applying a dedicated software algorithm. The chamber of interest (LA in the case of atrial fibrillation ablation) can then be used during the actual ablation procedure. LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle.
Figure 13 Image integration. The upper left image demonstrates the conventional electroanatomical map with the reconstructed anatomy of the LA. The blue, purple, red and green “tubes” represent the pulmonary veins. The lower left image shows the segmented LA derived from MSCT (see also fig 12). With the use of dedicated software, the CartoMerge Image Integration Module integrates both modalities.
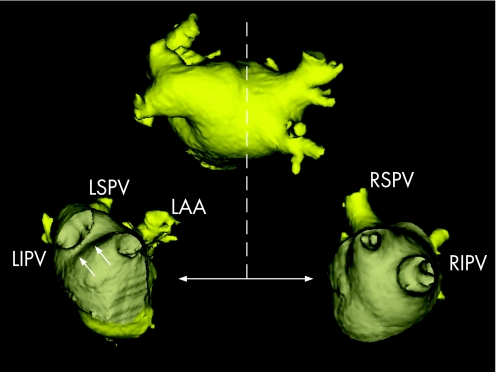
Figure 14 After the fusion process, the “real” anatomy provided by the MSCT scan can be used to guide the actual catheter ablation. With the use of dedicated tools, such as a “clipping plane” (represented by the dotted line), the ostium of the left‐sided pulmonary veins (lower left image) and the right‐sided pulmonary veins (lower right image) can be visualised. The relationship between the ablation catheter and the ostium of the pulmonary veins can be monitored constantly. In this patient, a “common ostium” of the left‐sided pulmonary veins is present. The white arrows on the lower left image indicate the ridge between the left‐sided pulmonary veins and the left atrial appendage (LAA). LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Recently, Kistler et al.54 compared 47 patients treated using conventional mapping alone with 47 patients treated using MSCT image integration. In the image integration group, fluoroscopy times were significantly shorter (49±27 minutes vs 62±26 minutes, p<0.05) and the number of patients with maintenance of sinus rhythm without anti‐arrhythmic medication was significantly higher in the image integration group (83% vs 60%, p<0.05).54 However, these data have to be confirmed in larger randomised trials.
One of the limitations of the new image integration technique is the time interval between the MSCT/MRI scan and the actual ablation procedure. Obviously, differences in fluid status, heart rate or rhythm are present between the two procedures, and may result in errors in the image fusion process. Furthermore, the accuracy of the image integration process may be affected by breathing during data acquisition and during the ablation procedure. In addition, variation of pulmonary vein location throughout the cardiac cycle may decrease the accuracy.56 Nonetheless, various studies have demonstrated the accuracy and value of image integration systems in guiding AF ablation procedures. 52,53,54,55 By merging online acquired electrophysiological data with detailed anatomical information, these image integration systems are valuable tools in the invasive treatment of patients with AF.
Summary
Assessment of LA size, anatomy and function is important in various clinical settings and can be performed with different imaging techniques. The assessment of LA size provides important prognostic information and is routinely performed with transthoracic echocardiography. Information on regional LA function can also be provided by transthoracic echocardiography and is important in the setting of treatment of AF. Catheter ablation procedures for AF require accurate imaging of the LA and surrounding structures. Intracardiac echocardiography, MSCT and MRI provide detailed information on LA and pulmonary vein anatomy. New image integration systems allow the online use of pre‐procedural acquired images and may facilitate ablation procedures and potentially improve the outcome.
Acknowledgements
The assistance of Jos Westenberg and Nina Ajmone Marsan in preparing the figures is gratefully acknowledged.
Abbreviations
AF - atrial fibrillation
ICE - intracardiac echocardiography
LA - left atrium
MSCT - multi‐slice CT
Footnotes
Competing interests: None.
References
- 1.Tsang T S, Barnes M E, Bailey K R.et al Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc 200176467–475. [DOI] [PubMed] [Google Scholar]
- 2.Pritchett A M, Jacobsen S J, Mahoney D W.et al Left atrial volume as an index of left atrial size: a population‐based study. J Am Coll Cardiol 2003411036–1043. [DOI] [PubMed] [Google Scholar]
- 3.Tsang T S, Abhayaratna W P, Barnes M E.et al Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol 2006471018–1023. [DOI] [PubMed] [Google Scholar]
- 4.Abhayaratna W P, Seward J B, Appleton C P.et al Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 2006472357–2363. [DOI] [PubMed] [Google Scholar]
- 5.Pritchett A M, Mahoney D W, Jacobsen S J.et al Diastolic dysfunction and left atrial volume: a population‐based study. J Am Coll Cardiol 20054587–92. [DOI] [PubMed] [Google Scholar]
- 6.Douglas P S. The left atrium: a biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol 2003421206–1207. [DOI] [PubMed] [Google Scholar]
- 7.Vaziri S M, Larson M G, Benjamin E J.et al Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation 199489724–730. [DOI] [PubMed] [Google Scholar]
- 8.Psaty B M, Manolio T A, Kuller L H.et al Incidence of and risk factors for atrial fibrillation in older adults. Circulation 1997962455–2461. [DOI] [PubMed] [Google Scholar]
- 9.Sanfilippo A J, Abascal V M, Sheehan M.et al Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 199082792–797. [DOI] [PubMed] [Google Scholar]
- 10.Dittrich H C, Pearce L A, Asinger R W.et al Left atrial diameter in nonvalvular atrial fibrillation: an echocardiographic study. Stroke Prevention in Atrial Fibrillation Investigators. Am Heart J 1999137494–499. [DOI] [PubMed] [Google Scholar]
- 11.Tsang T S, Barnes M E, Gersh B J.et al Prediction of risk for first age‐related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol 2003421199–1205. [DOI] [PubMed] [Google Scholar]
- 12.Osranek M, Bursi F, Bailey K R.et al Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three‐decade follow‐up. Eur Heart J 2005262556–2561. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin E J, D'Agostino R B, Belanger A J.et al Left atrial size and the risk of stroke and death: The Framingham Heart Study. Circulation 199592835–841. [DOI] [PubMed] [Google Scholar]
- 14.Fuster V, Ryden L E, Cannom D S.et al ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol 200648854–906. [DOI] [PubMed] [Google Scholar]
- 15.Ho S Y, Cabrera J A, Tran V H.et al Architecture of the pulmonary veins: relevance to radiofrequency ablation. Heart 200186265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jongbloed M R, Bax J J, Lamb H J.et al Multislice computed tomography versus intracardiac echocardiography to evaluate the pulmonary veins before radiofrequency catheter ablation of atrial fibrillation: a head‐to‐head comparison. J Am Coll Cardiol 200545343–350. [DOI] [PubMed] [Google Scholar]
- 17.Wood M A, Wittkamp M, Henry D.et al A comparison of pulmonary vein ostial anatomy by computerized tomography, echocardiography, and venography in patients with atrial fibrillation having radiofrequency catheter ablation. Am J Cardiol 20049349–53. [DOI] [PubMed] [Google Scholar]
- 18.Mansour M, Holmvang G, Sosnovik D.et al Assessment of pulmonary vein anatomic variability by magnetic resonance imaging: implications for catheter ablation techniques for atrial fibrillation. J Cardiovasc Electrophysiol 200415387–393. [DOI] [PubMed] [Google Scholar]
- 19.Hirata T, Wolfe S B, Popp R L.et al Estimation of left atrial size using ultrasound. Am Heart J 19697843–52. [DOI] [PubMed] [Google Scholar]
- 20.Lang R M, Bierig M, Devereux R B.et al Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005181440–1463. [DOI] [PubMed] [Google Scholar]
- 21.Lester S J, Ryan E W, Schiller N B.et al Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol 199984829–832. [DOI] [PubMed] [Google Scholar]
- 22.Ujino K, Barnes M E, Cha S S.et al Two‐dimensional echocardiographic methods for assessment of left atrial volume. Am J Cardiol 2006981185–1188. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins C, Bricknell K, Marwick T H. Use of real‐time three‐dimensional echocardiography to measure left atrial volume: comparison with other echocardiographic techniques. J Am Soc Echocardiogr 200518991–997. [DOI] [PubMed] [Google Scholar]
- 24.Maddukuri P V, Vieira M L, DeCastro S.et al What is the best approach for the assessment of left atrial size? Comparison of various unidimensional and two‐dimensional parameters with three‐dimensional echocardiographically determined left atrial volume. J Am Soc Echocardiogr 2006191026–1032. [DOI] [PubMed] [Google Scholar]
- 25.Rodevan O, Bjornerheim R, Ljosland M.et al Left atrial volumes assessed by three‐ and two‐dimensional echocardiography compared to MRI estimates. Int J Card Imaging 199915397–410. [DOI] [PubMed] [Google Scholar]
- 26.Block M, Hourigan L, Bellows W H.et al Comparison of left atrial dimensions by transesophageal and transthoracic echocardiography. J Am Soc Echocardiogr 200215143–149. [DOI] [PubMed] [Google Scholar]
- 27.Klein A L, Murray R D, Grimm R A. Role of transesophageal echocardiography‐guided cardioversion of patients with atrial fibrillation. J Am Coll Cardiol 200137691–704. [DOI] [PubMed] [Google Scholar]
- 28.Troughton R W, Asher C R, Klein A L. The role of echocardiography in atrial fibrillation and cardioversion. Heart 2003891447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jongbloed M R, Schalij M J, Zeppenfeld K.et al Clinical applications of intracardiac echocardiography in interventional procedures. Heart 200591981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrouche N F, Martin D O, Wazni O.et al Phased‐array intracardiac echocardiography monitoring during pulmonary vein isolation in patients with atrial fibrillation: impact on outcome and complications. Circulation 20031072710–2716. [DOI] [PubMed] [Google Scholar]
- 31.Verma A, Marrouche N F, Yamada H.et al Usefulness of intracardiac Doppler assessment of left atrial function immediately post‐pulmonary vein antrum isolation to predict short‐term recurrence of atrial fibrillation. Am J Cardiol 200494951–954. [DOI] [PubMed] [Google Scholar]
- 32.Rotter M, Sanders P, Jais P.et al Prospective validation of phased array intracardiac echocardiography for the assessment of atrial mechanical function during catheter ablation of atrial fibrillation. Heart 200692407–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamanaka K, Fujita M, Doi K.et al Multislice computed tomography accurately quantifies left atrial size and function after the MAZE procedure. Circulation 2006114(Suppl 1)I5–I9. [DOI] [PubMed] [Google Scholar]
- 34.Jongbloed M R, Dirksen M S, Bax J J.et al Atrial fibrillation: multi‐detector row CT of pulmonary vein anatomy prior to radiofrequency catheter ablation: initial experience. Radiology 2005234702–709. [DOI] [PubMed] [Google Scholar]
- 35.Anderson J L, Horne B D, Pennell D J. Atrial dimensions in health and left ventricular disease using cardiovascular magnetic resonance. J Cardiovasc Magn Reson 20057671–675. [DOI] [PubMed] [Google Scholar]
- 36.Kircher B, Abbott J A, Pau S.et al Left atrial volume determination by biplane two‐dimensional echocardiography: validation by cine computed tomography. Am Heart J 1991121(3 Pt 1)864–871. [DOI] [PubMed] [Google Scholar]
- 37.Marwick T H. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol 2006471313–1327. [DOI] [PubMed] [Google Scholar]
- 38.Thomas L, Levett K, Boyd A.et al Changes in regional left atrial function with aging: evaluation by Doppler tissue imaging. Eur J Echocardiogr 2003492–100. [DOI] [PubMed] [Google Scholar]
- 39.Quintana M, Lindell P, Saha S K.et al Assessment of atrial regional and global electromechanical function by tissue velocity echocardiography: a feasibility study on healthy individuals. Cardiovasc Ultrasound 200534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sirbu C, Herbots L, D'hooge J.et al Feasibility of strain and strain rate imaging for the assessment of regional left atrial deformation: a study in normal subjects. Eur J Echocardiogr 20067199–208. [DOI] [PubMed] [Google Scholar]
- 41.Di Salvo G, Caso P, Lo P R.et al Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent‐onset lone atrial fibrillation: a color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation 2005112387–395. [DOI] [PubMed] [Google Scholar]
- 42.Thomas L, McKay T, Byth K.et al Abnormalities of left atrial function after cardioversion: an atrial strain rate study. Heart 20079389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan I A. Atrial stunning: basics and clinical considerations. Int J Cardiol 200392113–128. [DOI] [PubMed] [Google Scholar]
- 44.Stefanadis C, Dernellis J, Toutouzas P. A clinical appraisal of left atrial function. Eur Heart J 20012222–36. [DOI] [PubMed] [Google Scholar]
- 45.Tops L F, Bax J J, Zeppenfeld K.et al Effect of radiofrequency catheter ablation for atrial fibrillation on left atrial cavity size. Am J Cardiol 2006971220–1222. [DOI] [PubMed] [Google Scholar]
- 46.Reant P, Lafitte S, Jais P.et al Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation 20051122896–2903. [DOI] [PubMed] [Google Scholar]
- 47.Tsao H M, Wu M H, Huang B H.et al Morphologic remodeling of pulmonary veins and left atrium after catheter ablation of atrial fibrillation: insight from long‐term follow‐up of three‐dimensional magnetic resonance imaging. J Cardiovasc Electrophysiol 2005167–12. [DOI] [PubMed] [Google Scholar]
- 48.Marom E M, Herndon J E, Kim Y H.et al Variations in pulmonary venous drainage to the left atrium: implications for radiofrequency ablation. Radiology 2004230824–829. [DOI] [PubMed] [Google Scholar]
- 49.Wongcharoen W, Tsao H M, Wu M H.et al Morphologic characteristics of the left atrial appendage, roof, and septum: implications for the ablation of atrial fibrillation. J Cardiovasc Electrophysiol 200617951–956. [DOI] [PubMed] [Google Scholar]
- 50.Ren J F, Callans D J, Schwartzman D.et al Changes in local wall thickness correlate with pathologic lesion size following radiofrequency catheter ablation: an intracardiac echocardiographic imaging study. Echocardiography 200118503–507. [DOI] [PubMed] [Google Scholar]
- 51.Gepstein L, Hayam G, Ben Haim S A. A novel method for nonfluoroscopic catheter‐based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation 1997951611–1622. [DOI] [PubMed] [Google Scholar]
- 52.Dong J, Calkins H, Solomon S B.et al Integrated electroanatomic mapping with three‐dimensional computed tomographic images for real‐time guided ablations. Circulation 2006113186–194. [DOI] [PubMed] [Google Scholar]
- 53.Tops L F, Bax J J, Zeppenfeld K.et al Fusion of multislice computed tomography imaging with three‐dimensional electroanatomic mapping to guide radiofrequency catheter ablation procedures. Heart Rhythm 200521076–1081. [DOI] [PubMed] [Google Scholar]
- 54.Kistler P M, Rajappan K, Jahngir M.et al The impact of CT image integration into an electroanatomic mapping system on clinical outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2006171093–1101. [DOI] [PubMed] [Google Scholar]
- 55.Heist E K, Chevalier J, Holmvang G.et al Factors affecting error in integration of electroanatomic mapping with CT and MR imaging during catheter ablation of atrial fibrillation. J Interv Card Electrophysiol 20061721–27. [DOI] [PubMed] [Google Scholar]
- 56.Noseworthy P A, Malchano Z J, Ahmed J.et al The impact of respiration on left atrial and pulmonary venous anatomy: implications for image‐guided intervention. Heart Rhythm 200521173–1178. [DOI] [PubMed] [Google Scholar]