Abstract
Background
Gender differences in management and outcomes have been reported in acute coronary syndrome (ACS).
Objectives
To assess such gender differences in a Swiss national registry.
Methods
20 290 patients with ACS enrolled in the AMIS Plus Registry from January 1997 to March 2006 by 68 hospitals were included in a prospective observational study. Data on patients' characteristics, diagnoses, procedures, complications and outcomes were recorded. Odds ratios (ORs) of in‐hospital mortality were calculated using logistic regression models.
Results
5633 (28%) patients were female and 14 657 (72%) male. Female patients were older than men (mean (SD) age 70.9 (12.1) vs 63.4 (12.9) years; p<0.001), had more comorbidities and came to hospital later. They underwent percutaneous coronary intervention (PCI) less frequently (OR = 0.65; 95% CI 0.61 to 0.69) and their unadjusted in‐hospital mortality was higher overall (10.7% vs 6.3%; p<0.001) and in those who underwent PCI (3.0% vs 4.2%; p = 0.018). Mortality differences between women and men disappeared after adjustments for other predictors (adjusted OR (aOR) for women vs men: 1.09; 95% CI 0.95 to 1.25), except in women aged 51–60 years (aOR = 1.78; 95% CI 1.04 to 3.04). However, even after adjustments, female gender remained significantly associated with a lower probability of undergoing PCI (OR = 0.70; 95% CI 0.64 to 0.76).
Conclusions
The analysis showed gender differences in baseline characteristics and in the rate of PCI in patients admitted for ACS in Swiss hospitals between 1997 and 2006. Reasons for the significant underuse of PCI in women, and a slightly higher in‐hospital mortality in the 51–60 year age group, need to be investigated further.
Coronary artery disease and, in particular, acute coronary syndrome (ACS), is the leading cause of mortality and morbidity in the Western world, in both women and men.
The benefits of reperfusion treatment for patients with ACS have been well established and it has become standard treatment for both women and men with ST‐segment elevation acute coronary syndrome (STE‐ACS); however, there is variation in the method of reperfusion chosen, and in which patients are considered eligible.1 Controversies also exist about the type and the time of reperfusion and about its outcomes in patients presenting with unstable angina or non‐ST‐segment elevation (NSTE‐ACS).
It has also been shown that women with acute myocardial infarction (AMI) are less likely than men to undergo reperfusion treatment,2,3 and that there is a lack of awareness of risk among women.4 In addition, there are conflicting data from randomised trials about the benefit of early invasive treatment in women.5,6,7 Differences in survival between men and women reported in some studies may not only reflect gender bias in management, but also differences in coronary anatomy, age and comorbidities. In the CADILLAC Trial, women had higher mortality than men after interventional treatment for AMI, which the authors attributed to smaller body surface area and more comorbidities.3 On the contrary, other authors have suggested that the higher mortality seen in women after an AMI might be explained by less aggressive treatment,8 and if women had access to the same quality of care as men, their survival would be the same.9 Finally, the results of outcome studies in unselected patients suggest that gender is not an independent predictor of mortality after percutaneous coronary intervention (PCI)2,10 and that improvement in prognosis associated with reperfusion treatment is independent from it.10,11,12,13 The data of 3100 female patients enrolled in the Euro Heart Survey ACS showed that female gender in the “real world” was not independently associated with worse in‐hospital mortality, irrespective of the type of ACS.14 The authors interestingly emphasised the need to evaluate outcomes of ACS in surveys or registries, rather than from data derived from clinical trials.14 This suggestion, however, did not solve the controversy since, in the New York angioplasty registry, in‐hospital mortality for female patients undergoing angioplasty after having reached hospital within 6 hours was 9.04% vs 4.42% for male (p<0.001) for the years 1993–6.15
Thus, the aim of this study was to assess outcomes in unselected female and male patients admitted between 1997 and 2006 for ACS in Swiss hospitals and to put these results in the perspective of their baseline characteristics, comorbidities and management.
Patients and methods
The AMIS Plus Registry
In 1997, the Swiss Societies of Cardiology, Internal Medicine and Intensive Care initiated a nationwide prospective registry to assess diagnostic and therapeutic measures in patients with acute myocardial infarction in Switzerland (AMIS). Academic and non‐academic hospitals participate voluntarily and provide blinded data to a data centre through an internet‐ or paper‐based questionnaire of 140 questions. The data centre controls and checks data for plausibility and crosschecks in case of queries. AMIS Plus is an industry‐sponsored project, but its supporting institutions do not play any part in the design of the registry, data collection, analysis or interpretation. The project is led by a steering committee comprising members of the founding societies. The registry was approved by the Over‐regional Ethical Committee for Clinical Studies and the Swiss Board for Data Security.
Patients
The AMIS Plus Registry documented data from 20 549 patients admitted to hospital for an acute coronary syndrome between January 1997 and March 2006. The AMIS Plus Registry included all patients with ACS: AMI, defined by characteristic symptoms and or ECG changes and enzyme rises (total creatine kinase or creatine kinase MB fraction) at least twice the upper limit or normal; ACS with minimal necrosis (symptoms or ECG changes compatible with ACS and cardiac enzymes lower than twice the upper limit of normal range and positive troponins); and unstable angina (symptoms or ECG changes compatible with ACS and normal cardiac enzymes). For this analysis, all patients with valid data on initial ECG and reperfusion were included. Patients included in this analysis were categorised as having STE‐ACS or NSTE‐ACS based on the initial ECG findings. Classification of STE‐ACS included evidence of ACS as above and ST‐segment elevation and/or new left bundle branch block (LBBB) on the initial ECG. NSTE‐ACS included patients with ischaemic symptoms, ST‐segment depression or T‐wave abnormalities in the absence of ST elevation on the initial ECG.
Statistical analysis
Data are presented as percentages of valid cases for discrete variables and as mean (SD) and/or median for continuous variables. Differences in baseline characteristics were compared using the Student t test and χ2 test. User‐defined missing values are treated as missing. Statistics for each table are based on all cases with valid data in the specified ranges for all variables in each table. Odds ratios (ORs) of in‐hospital mortality were calculated using logistic regression models. The following set of variables, available at hospital admission were included: age for each additional year, history of coronary heart disease, arterial hypertension, dyslipidaemia, diabetes, current smoking, Killip class at hospital admission (Killip class I as reference category), delay between symptom onset and admission to hospital >6 hours; LBBB, ST‐segment elevation, ST‐segment depression and Q waves on initial electrocardiogram, body mass index, heart rate, systolic blood pressure and PCI. Separate univariate logistical models were first adjusted for each variable and then backward elimination with a significance level of 0.05 was performed. ORs were simultaneously adjusted for all other predictors included in the multivariate logistic regression model. SPSS, version 13.0 (Chicago, Illinois, USA) was used for all statistical analyses.
Results
From 20 549 patients admitted for ACS and enrolled in the AMIS Plus Registry, 20 290 patients were available for this analysis: 5633 (28%) women and 14 657 (72%) men. Excluded were patients with missing data on initial ECG (n = 126) and reperfusion (n = 133).
Table 1 gives baseline characteristics of the 20 290 patients.
Table 1 Baseline characteristics of patients with acute coronary syndrome (ACS) (n = 20 290).
| Characteristics | Men | Women | p Value |
|---|---|---|---|
| Number of patients (%) | 14 657 (72) | 5633 (38) | |
| Age (years) | |||
| Min–max | 22–100 | 22–99 | <0.001 |
| Mean (SD) | 63.4 (12.9) | 70.9 (12.1) | |
| Median | 64 | 73 | |
| Type of ACS | |||
| ST‐segment elevation and/or new LBBB (%) | 60 | 58 | 0.20 |
| Non‐ST elevation/unstable angina (%) | 40 | 42 | 0.10 |
| Delay (hours), median (interquartile range) | 4:00 (1:55–11:45) | 5:00 (2:15–14:00) | <0.001 |
| Cardiopulmonary resuscitation (%) | 3.8 | 3.3 | 0.098 |
| Cardioversion/defibrillation (%) | 3.5 | 2.9 | 0.042 |
| Symptoms at admission | |||
| Pain (%) | 82.3 | 79.7 | <0.001 |
| Dyspnoea (%) | 23.0 | 31.7 | <0.001 |
| Systolic blood pressure (mm Hg), mean (SD) | 135 (27) | 137 (29) | <0.001 |
| Diastolic blood pressure (mm Hg), mean (SD) | 80 (18) | 78 (18) | <0.001 |
| Heart rate (beats/min), mean (SD) | 78 (21) | 82 (23) | <0.001 |
| Heart rhythm | |||
| Sinus rhythm (%) | 92.2 | 89.7 | 0.732 |
| Atrial fibrillation (%) | 4.7 | 6.9 | <0.001 |
| ECG at admission | |||
| ST elevation (%) | 56.7 | 53.7 | <0.001 |
| Q waves (%) | 23.8 | 21.8 | 0.020 |
| ST depression (%) | 24.7 | 26.0 | 0.065 |
| T‐wave changes (%) | 26.4 | 27.6 | 0.083 |
| LBBB (%) | 5.2 | 6.4 | 0.001 |
| RBBB (%) | 4.4 | 3.6 | 0.013 |
| Killip class | |||
| I (%) | 78.0 | 69.6 | <0.001 |
| II (%) | 15.6 | 21.3 | <0.001 |
| III (%) | 4.1 | 6.8 | <0.001 |
| IV (%) | 2.3 | 2.3 | 1.000 |
| Past medical history | |||
| Coronary artery disease (%) | 39.6 | 37.4 | 0.070 |
| Hypertension (%) | 51.7 | 65.2 | <0.001 |
| Dyslipidaemia (%) | 59.4 | 56.2 | <0.001 |
| Diabetes (%) | 18.7 | 23.7 | <0.001 |
| Smoking (current) (%) | 43.4 | 25.0 | <0.001 |
| Overweight (BMI ⩾25) (%) | 66.8 | 55.3 | <0.001 |
BMI, body mass index; LBBB, left bundle branch block; RBBB, right bundle branch block.
Female patients were older than male patients and more often had a history of hypertension or diabetes, but less frequently of dyslipidaemia; they were less frequently overweight or smokers. Female patients came to hospital later (median difference: 60 minutes), were more frequently dyspnoeic and in Killip classes II/III. Their admission ECG more often showed ST‐segment depression, LBBB or atrial fibrillation. The same proportion of women and men had a diagnosis of STE‐ACS and NSTE‐ACS. Table 2 lists drug treatment and reperfusion strategies.
Table 2 Drug treatment and reperfusion strategies (n = 20 290).
| Men | Women | p Value | |
|---|---|---|---|
| Number of patients | 14 657 | 5633 | |
| Aspirin (%) | 94.3 | 92.2 | <0.001 |
| Clopidogrel (%) | 44.7 | 36.1 | <0.001 |
| GPIIb/IIIa antagonist (%) | 36.9 | 27.1 | <0.001 |
| Unfractionated heparin (%) | 73.0 | 69.4 | <0.001 |
| LMWH (%) | 32.2 | 34.6 | 0.006 |
| β Blocker (%) | 73.6 | 67.3 | <0.001 |
| ACE inhibitor (%) | 39.4 | 39.4 | 1.000 |
| Angiotensin antagonist (%) | 4.5 | 6.0 | <0.001 |
| Calcium channel blocker (%) | 6.5 | 7.5 | 0.013 |
| Nitrate (%) | 67.1 | 67.4 | 0.657 |
| Lipid‐lowering drug (%) | 73.1 | 63.6 | <0.001 |
| Thrombolysis (%) | 18.7 | 15.2 | <0.001 |
| PCI (%) | 36.6 | 27.2 | <0.001 |
LMWH, low molecular weight heparin; PCI, percutaneous coronary intervention.
Significantly fewer women than men received aspirin, clopidogrel, GPIIb/IIIa antagonists, β blockers and angiotensin‐converting enzyme (ACE) inhibitors. In addition, 1805/3287 (54.9%) women with STE‐ACS underwent any type of reperfusion treatment compared with 6150/8859 (69.4%) men (p<0.001).
Women underwent PCI less often than men: of the 3287 women presenting with STE‐ACS, 1016 (30.9%) underwent primary PCI, as well as 516 (22.0%) of the 2346 women with NSTE‐ACS; by contrast, 3572 (40.3%; p<0.001) of the 8859 male STE‐patients with ACS underwent primary PCI, as well as 1793 (30.9%, p<0.001) of the 5798 men with NSTE‐ACS. These differences between women and men persisted after adjustments. Overall, female gender was an independent factor for undergoing PCI less frequently. Table 3 shows the adjusted ORs of undergoing PCI for gender, as well as for all other significant variables.
Table 3 Predictors for undergoing primary percutaneous coronary intervention by multivariable analysis (n = 13 217)*.
| Predictors | Odds ratio (95% CI for OR) | P‐value |
|---|---|---|
| Age (for each additional year) | 0.98 (0.97 to 0.98) | <0.001 |
| Female gender | 0.70 (0.64 to 0.76) | <0.001 |
| Killip class II | 0.43 (0.37 to 0.46) | <0.001 |
| Killip class III | 0.28 (0.22 to 0.35) | <0.001 |
| Killip class IV | 1.05 (0.82 to 1.34) | 0.698 |
| Delay >6 hours | 1.20 (1.11 to 1.29) | <0.001 |
| History of CAD | 0.68 (0.63 to 0.74) | <0.001 |
| History of dyslipidaemia | 1.20 (1.12 to 1.30) | <0.001 |
| ST‐segment elevation | 1.77 (1.64 to 1.91) | <0.001 |
| LBBB | 0.58 (0.47 to 0.71) | <0.001 |
CAD, coronary artery disease; LBBB, left bundle branch block.
*7073 Patients could not be included in this analysis because they had missing values for some of the adjustment variables.
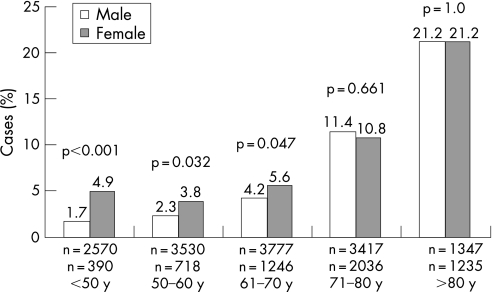
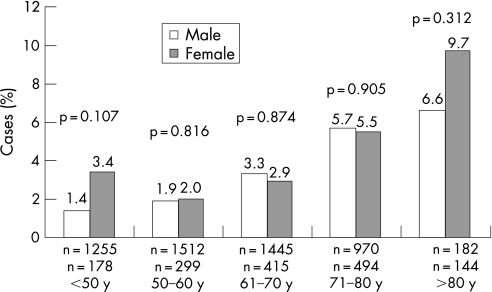
Unadjusted in‐hospital mortality was higher in female patients (601/5633; 10.7%) than in male patients (925/14 657; 6.3%, p<0.001). Mortality in women with STE‐ACS was 13.0% and in women with NSTE‐ACS 7.5%, while it was 7.2% (p<0.001) and 4.9% (p<0.001), respectively, for men. In‐hospital mortality was also higher in women who underwent PCI (65/1532; 4.2%) than in men (160/5365; 3.0%, p = 0.018). Differences in in‐hospital mortality between all men and women were mostly due to younger patients: in‐hospital mortality according to age groups showed that significantly more women than men died only at age <50 (fig 1). Although not significant, the same trend was observed for patients who underwent PCI (fig 2).
Figure 1 In‐hospital mortality of patients with acute coronary syndrome according to age groups (n = 20 266).
Figure 2 In‐hospital mortality of patients with acute coronary syndrome who underwent percutaneous coronary intervention according to age groups (n = 6894).
However, after adjustments for all differences between women and men by multivariable analysis, female gender was no longer significantly associated with greater in‐hospital mortality, within age categories and overall, except for the 51–60 years of age category, where the odds ratios of mortality reached borderline statistical significance.
Table 4 lists unadjusted, as well as adjusted odds ratios of in‐hospital mortality for women by 10‐year age categories, as well as overall.
Table 4 In‐hospital mortality of all patients (n = 20 266)* with acute coronary syndrome according to age categories.
| Age categories (years) | ORs (95% CI for OR) | |
|---|---|---|
| Female unadjusted | Female adjusted† | |
| ⩽50 (n = 2960) | 2.94 (1.70 to 5.09) | 1.66 (0.69–4.05) |
| 51–60 (n = 4248) | 1.66 (1.07 to 2.59) | 1.78 (1.04–3.04) |
| 61–70 (n = 5023) | 1.35 (1.02 to 1.81) | 1.33 (0.93–1.91) |
| 71–80 (n = 5453) | 1.05 (0.88 to 1.25) | 1.07 (0.86–1.33) |
| >80 (n = 2582) | 1.00 (0.83 to 1.21) | 0.91 (0.72–1.15) |
| All age categories | 1.77 (1.59 to 1.98) | 1.44 (1.26–1.65) |
Male (reference) = 1.
*24 Patients could not be included in this analysis because they had missing values for some of the adjustment variables.
†For Killip class, history of diabetes, hypertension, dyslipidaemia, ST elevation on initial ECG and percutaneous coronary intervention.
Table 5 lists all variables significantly associated with in‐hospital mortality.
Table 5 Predictors of in‐hospital mortality upon admission by multivariable analysis (n = 20 266)*.
| Predictors | Odds ratio (95% CI for OR) | p Value |
|---|---|---|
| Female gender | 1.09 (0.95 to 1.25) | 0.244 |
| Age (for each additional year) | 1.06 (1.05 to 1.07) | <0.001 |
| Killip class II | 2.38 (2.04 to 2.78) | <0.001 |
| Killip class III | 4.55 (3.71 to 5.59) | <0.001 |
| Killip class IV | 24.5 (19.0 to 31.5) | <0.001 |
| History of diabetes | 1.27 (1.09 to 1.47) | 0.002 |
| History of dyslipidaemia | 0.73 (0.64 to 0.83) | <0.001 |
| ST segment elevation | 1.69 (1.47 to 1.94) | <0.001 |
| LBBB | 1.75 (1.42 to 2.15) | <0.001 |
| PCI | 0.52 (0.44 to 0.63) | <0.001 |
LBBB, left bundle branch block; PCI, percutaneous coronary intervention.
*24 Patients could not be included in this analysis because they had missing values for some of the listed variables.
Similarly, female gender was not significantly associated anymore with higher odds of in‐hospital mortality in patients who underwent PCI (table 6) after adjustments. When men and women were looked at separately, the same variables were significant predictors of in‐hospital mortality, except that history of diabetes was not significant anymore for both female (p = 0.066) and male patients (p = 0.052).
Table 6 Predictor of in‐hospital mortality upon admission by multivariable analysis in patients who underwent percutaneous coronary intervention (n = 6659)*.
| Predictor | Odds ratio (95% CI for OR) | p Value |
|---|---|---|
| Female gender | 1.11 (0.79 to 1.56) | 0.554 |
| Age (for each additional year) | 1.05 (1.04 to 1.07) | <0.001 |
| Killip class II | 3.16 (2.13 to 4.70) | <0.001 |
| Killip class III | 8.97 (5.03 to 15.99) | <0.001 |
| Killip class IV | 36.7 (24.7 to 54.4) | <0.001 |
| History of diabetes | 1.53 (1.09 to 2.14) | 0.015 |
*238 Patients could not be included in this analysis because they had missing values for some of the listed variables.
In addition, the differences in mortality between female and male patients who underwent PCI were not significant when categorised by types of ACS; STE‐ACS: 5.0% for women vs 3.4% for men (p = 0.019); and NSTE‐ACS: 2.7% vs 2.2% (p = 0.504).
Major adverse cardiac events (reinfarction, stroke and death) occurred in 13.9% of female patients and in 8.8% of male patients (p<0.001). Cardiogenic shock occurred in 10.2% of women and 7.2% of men (p<0.001), reinfarction 3.8% in women and 2.6% men (p<0.001), and cerebrovascular events in 1.4% women and 0.9% men (p = 0.003). These differences in the occurrence of major cardiac events in women were not significant once adjusted for differences in clinical characteristics and PCI (OR = 1.11; 95% CI 0.99 to 1.26; p = 0.08).
Discussion
Data from the Swiss national registry AMIS Plus showed that there were not only differences in baseline characteristics between men and women admitted for ACS in Swiss hospitals between 1997 and 2006 but also in their management, from drugs such as aspirin to PCI. Our data also showed that, in Switzerland, PCI has become the preferred treatment not only for STE‐ACS16 but also for NSTE‐ACS, in women as well as in men. Although performed less often than in men, women benefited similarly from PCI and it was associated with lower in‐hospital mortality, whether or not ACS was associated with ST‐segment elevation. Indeed, the unadjusted in‐hospital mortality of women with STE‐ACS was 13.0% and of women with NSTE‐ACS 7.5%, which was lower than the in‐hospital mortality for both genders in the National Registry of Myocardial Infarction 4 (14.3% for STE‐ACS and 12.5% in NSTE‐ACS).8
Our results confirm prior studies, which showed that women with AMI often did not receive the same interventional treatment as men,17 although women had similar or even better outcomes after PCI.18,19 Data from CRUSADE20 have shown that despite presenting with higher risk characteristics and having a higher in‐hospital risk, women with non‐ST‐segment elevation myocardial infarction (NSTEMI) were treated less aggressively than men.8 Similar observations were made in Europe by Heer et al for both STEMI and NSTEMI.21,22
Other studies found no major difference in the management of men and women with unstable angina.23,24 In our study, female gender remained significantly associated with a lower probability of undergoing PCI, even after adjusting for the presence of STE or LBBB. The reasons for this underuse remain unclear.
Studies comparing outcomes of men and women with ACS have provided conflicting results and unconvincing explanations. Unadjusted comparisons of mortality after AMI have generally indicated that women have a poorer outcome than men,25,26 have less favourable near‐term outcomes after revascularisation procedures25 and that they are at increased risk for adverse outcomes.10,27,28 In the New York angioplasty registry, the in‐hospital mortality for all primary angioplasty patients between 1993 and 1996 was 5.81% overall but 9.04% in women.15 In some studies, female gender was a risk factor for long‐term mortality among patients who underwent primary angioplasty but not for short‐term mortality,15 whereas in other studies mortality was higher for women soon after PCI and before hospital discharge, mainly because of a higher rate of non‐cardiac death.29 The TACTICS TIMI‐18 trial showed a clear benefit of an early invasive approach in NSTE‐ACS regardless of gender,6 whereas in FRISC II and in RITA 3, the benefits of such an approach were seen only in men.7,30 More recently, it has been suggested that the difference in outcome between women and men treated with PCI had decreased and that the outcome in women had improved.12,31,32 Authors from the CADILLAC trial suggested that the higher mortality seen in women compared with men after interventional treatment for AMI might be explained by differences in body size and clinical risk factors.3 However, smaller target vessel size is associated with an increased risk of restenosis, but does not appear to be a predictor for mortality.28 Nevertheless, basic biological differences in response to AMI between men and women have also been advocated27,33 in addition to anatomical differences.34 It has also been suggested that there is a different pathophysiology of ACS in younger, but not older women.35
Studies on elderly patients with ACS have shown less aggressive treatment and higher mortality than in younger patients.36 However, gender differences in mortality were not obvious,25,37 and a recent analysis of the National Registry of Myocardial Infarction found that the excess risk of mortality for women was accentuated at an earlier age and tended to disappear in older patients.25,26,38 A higher 1‐year mortality was seen in women with AMI in the French USIC Registry, owing to a higher risk of death in women aged 30–67 years during the initial hospitalisation.39 However, another study showed a worse early outcome in elderly women with STE‐ACS compared with men after adjustment for comorbidities, whereas similar outcomes were noted among patients with NSTE‐ACS.40 Overall, our study showed similar outcomes for men and women after adjusting for clinical characteristics and ECG findings, whether or not PCI was included in the model. However, in‐hospital mortality of women aged 51–60 years remained slightly greater than that of men of the same age category, but this difference was of borderline significance. Whether it reflects true differences linked with suboptimal management or only residual confounders cannot be answered from our current data.
Our study has some limitations common to all registries. First, participation in the AMIS Plus Registry is voluntary; therefore, we could not verify whether consecutive patients with ACS were included by participating sites, or if selection biases occurred. Although 68 (64%) of the 106 Swiss hospitals treating ACS at the time of the study were included in the AMIS Plus Registry, the number of participating centres varied during the study period; thus, participating hospitals and recruited patients may not be entirely representative of all hospitals and all patients with ACS in the country. Nevertheless, the AMIS Plus database is of substantial size for a small country like Switzerland and represents hospitals of various magnitude and equipment, making it more representative of current practice patterns than previous single‐site databases or randomised trials. Inaccuracies in data entry cannot totally be ruled out and may thus have created unrecognised biases; although individual on‐site auditing at the participating centres was performed sporadically, but not systematically, data questionnaires were continuously and carefully checked by the data management centre, and incomplete questionnaires were queried as needed. In the logistic regression analyses, the burden of comorbidity was limited to history of coronary artery disease, hypertension, dyslipidaemia and diabetes. In the whole dataset, there were no significant gender differences in the proportion of cerebrovascular diseases (203/3034 female vs 505/8015 male), or neoplasm 154/3034 female vs 354/8015 male patients). Nevertheless, no summary variable reflecting comorbidities was available for all patients in the dataset. Finally, our study concentrated on in‐hospital mortality and there are no follow‐up data available to allow longer‐term comparison of outcomes.
In summary, data from the Swiss registry AMIS Plus showed that there were differences in baseline characteristics and in the management of women and men admitted for ACS in Swiss hospitals. In particular, PCI was performed less often in women than in men. Overall, in‐hospital mortality was similar for women and men after adjustments, but in women aged 51–60 years, mortality remained slightly greater than that for men. The reasons for the significant underuse of PCI in women need to be further investigated, together with the management and outcome of younger women, who seem to be an unrecognised risk group.
Acknowledgements
We gratefully thank Professor Burkhardt Seifert from the Department of Biostatistics, Institute of Social and Preventive Medicine, Zurich, for his assistance with the statistical analysis.
The supporting institutions did not play any role in the design of the registry, data collection, analysis or interpretation.
Abbreviations
ACS - acute coronary syndrome
AMI - acute myocardial infarction
LBB - left bundle branch block
NSTE - non‐ST‐segment elevation
OR - odds ratio
PCI - percutaneous coronary intervention
STE - ST‐segment elevation
Appendix
Amis plus steering committee
P Erne, president, Lucerne, FW Amman, Zurich, O Bertel, Zurich, E Camenzind, Geneva, F Eberli, Zurich, M Essig, Zweisimmen, J‐M Gaspoz, Geneva, F Gutzwiller, Zurich, P Hunziker, Basel, M Maggiorini, Zurich, B Quartenoud, Fribourg, H Rickli, St Gallen, J‐C Stauffer, Lausanne, P Urban, Geneva, S Windecker, Bern
Amis plus participating centres
The following hospitals participated from 1997–2006 in the AMIS registry on which this report is based (in alphabetical order): Altdorf, Kantonsspital Altdorf: Dr R Simon, Altstätten, Kantonales Spital Altstätten: Dr P‐J Hangartner/Dr M Rhyner, Baden, Kantonsspital Baden: Dr M Neuhaus, Basel, Kantonsspital Basel: PD Dr P Hunziker, Basel, St Claraspital: Dr C Grädel, Bern, Inselspital: Prof B Meier/PD Dr S Windecker, Biel, Spitalzentrum Biel: Dr H Schläpfer, Brig‐Glis, Oberwalliser Kreisspital: Dr D Evéquoz, Bülach, Spital Bülach: Dr R Pampaluchi/Dr A Ciurea‐Löchel/Dr M Kruhl, Chur, Rätisches Kantons‐ und Regionalspital Chur: Dr P Müller, Chur, Kreuzspital: Dr V Wüscher/Dr R Jecker, Davos Platz, Spital Davos: Dr G Niedermaier, Dornach, Spital Dornach: Dr A Koelz, Flawil, Kantonales Spital Flawil: Dr T Langenegger, Frauenfeld, Kantonsspital Frauenfeld: Dr H‐P Schmid, Fribourg, Hôpital cantonal de Fribourg: Dr B Quartenoud, Frutigen, Spital Frutigen: Dr S Moser/Dr Kuengolt Bietenhard, Genève, Hôpitaux universitaires de Genève (HUG): Prof J‐M Gaspoz, Glarus, Kantonsspital Glarus: Dr W Wojtyna, Grenchen, Spital Grenchen: Dr P Schlup/Dr A Oestmann, Grosshöchstetten, Bezirksspital Grosshöchstetten: Dr C Simonin, Heiden, Kantonales Spital Heiden: Dr R Waldburger, Herisau, Kantonales Spital Herisau: Dr P Staub/Dr M Schmidli, Interlaken, Spital Interlaken: Dr P Sula/Dr Ph Furger, Jegenstorf, Spital Jegenstorf: Dr H Marty, Kreuzlingen, Herz‐Neuro‐Zentrum Bodensee: Dr K Weber, La Chaux‐de‐Fonds, Hôpital La Chaux‐de‐Fonds: Dr H Zender, Lachen, Regionalsspital Lachen: Dr I Poepping/Dr C Steffen, Langnau im Emmental, Regionalspital Emmental: Dr J Sollberger, Lugano, Cardiocentro Ticino: Dr G Pedrazzini, Luzern, Kantonsspital Luzern: Prof P Erne, Männedorf, Kreisspital Männedorf: Dr J von Meyenburg/Dr T Luterbacher, Martigny, Hôpital régional de Martigny: Dr B Jordan, Mendrisio, Ospedale regionale di Mendrisio: Dr A Pagnamenta, Meyrin, Hôpital de la Tour: PD Dr P Urban, Monthey, Hôpital du Chablais: Dr P Feraud, Montreux, Hôpital de Zone: Dr E Beretta, Moutier, Hôpital du Jura bernois: Dr C Stettler, Münsingen, Regionales Spital Zentrum Münsingen: Dr F Repond, Münsterlingen, Kantonsspital Münsterlingen: Dr F Widmer, Muri, Kreisspital für das Freiamt: Dr A Spillmann/Dr F Scheibe/Dr K Rudaz‐Schwaller, Nyon, Group Hosp Ouest lémanique: Dr R Polikar, Rheinfelden, Gesundheitszentrum Fricktal Regionalspital Rheinfelden: Dr H‐U Iselin, Rorschach, Kantonales Spital Rorschach: Dr M Pfister, Samedan, Spital Oberengadin: Dr P Egger, Sarnen, Kantonsspital Obwalden: Dr T Kaeslin, Schaffhausen, Kantonsspital Schaffhausen: Dr R Frey, Schlieren, Spital Limmattal: Dr B Risti/Dr V Stojanovic/Dr T Herren, Schwyz, Spital Schwyz: Dr P Eichhorn, Scuol, Ospidal d'Engiadina Bassa: Dr G Flury/Dr C Neumeier, Solothurn, Bürgerspital Solothurn: Dr P Hilti, St Gallen, Kantonsspital St Gallen: Dr W Angehrn/Dr H Rickli, Thun, Spital Thun: Dr U Stoller, Thusis, Krankenhaus Thusis: Dr U‐P Veragut, Uster, Spital Uster: Dr D Maurer/PD Dr J Muntwyler, Uznach, Kantonales Spital Uznach: Dr A Weber, Wädenswil, Schwerpunktspital Zimmerberg‐Horgen: Dr G Garzoli/Dr B Kälin, Wald, Spital Wald: Dr M Schneider, Walenstadt, Kantonales Spital Walenstadt: Dr H Matter/Dr D Schiesser, Wetzikon, GZO Spital Wetzikon: Dr M Graber, Winterthur, Kantonsspital Winterthur: Dr A Haller, Wolhlusen, Kantonales Spital Sursee‐Wolhusen: Dr M Peter, Zofingen, Spital Zofingen: Dr HJ Vonesch/Dr HJ Meier/Dr S Gasser, Zollikerberg, Spital Zollikerberg: Dr P Siegrist/Dr R Fatio, Zug, Zuger Kantonsspital: Prof M Vogt, Zürich, Universitätsspital Zürich: PD Dr F Eberli/PD Dr M Maggiorini, Zürich, Stadtspital Triemli: Prof O Bertel, Zürich, Stadtspital Waid: Dr M Brabetz/Dr S Christen.
Footnotes
Funding: The AMIS Plus Registry is funded by grants from (in alphabetical order): AstraZeneca, Switzerland; Bayer, Switzerland; Biotronik, Switzerland; Boehringer Ingelheim, Switzerland; Boston Scientific, Switzerland; Bristol‐Myers Squibb, Switzerland; Essex Chemie, Switzerland; GlaxoSmithKline, Switzerland; Guidant, Switzerland; Invatec, Switzerland; Johnson&Johnson/Cordis, Switzerland; Jomed, Switzerland; MCMmedsys, Switzerland; Medtronic, Switzerland; Mepha, Switzerland; A. Menarini, Switzerland; Merck, Switzerland; MSD, Switzerland; Pfizer, Switzerland; Rahn, Switzerland; Roche Pharma, Switzerland; Sanofi‐Aventis, Switzerland; Schering, Switzerland; Servier, Switzerland; St. Jude Medical, Switzerland; SPSS, Switzerland; Swiss Heart Foundation, Takeda Pharma, Switzerland and Vision Foundation, Switzerland.
Conflict of interest: None.
References
- 1.Eagle K A, Goodman S G, Avezum A.et al Practice variation and missed opportunities for reperfusion in ST‐segment‐elevation myocardial infarction: findings from the Global Registry of Acute Coronary Events (GRACE). Lancet 2002359373–377. [DOI] [PubMed] [Google Scholar]
- 2.Antoniucci D, Valenti R, Moschi G.et al Sex‐based differences in clinical and angiographic outcomes after primary angioplasty or stenting for acute myocardial infarction. Am J Cardiol 200187289–293. [DOI] [PubMed] [Google Scholar]
- 3.Lansky A J, Hochman J S, Ward P A.et al Percutaneous coronary intervention and adjunctive pharmacotherapy in women: a statement for healthcare professionals from the American Heart Association. Circulation 2005111940–953. [DOI] [PubMed] [Google Scholar]
- 4.Mosca L, Appel L J, Benjamin E J.et al Evidence‐based guidelines for cardiovascular disease prevention in women. Circulation 2004109672–693. [DOI] [PubMed] [Google Scholar]
- 5.Elkoustaf R A, Boden W E. Is there a gender paradox in the early invasive strategy for non ST‐segment elevation acute coronary syndromes? Eur Heart J 2004251559–1561. [DOI] [PubMed] [Google Scholar]
- 6.Cannon C P, Weintraub W S, Demopoulos L A.et al Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med 20013441879–1887. [DOI] [PubMed] [Google Scholar]
- 7.Lagerqvist B, Safstrom K, Stahle E.et al Is early invasive treatment of unstable coronary artery disease equally effective for both women and men? FRISC II Study Group Investigators. J Am Coll Cardiol 20013841–48. [DOI] [PubMed] [Google Scholar]
- 8.Blomkalns A L, Chen A Y, Hochman J S.et al Gender disparities in the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes: large‐scale observations from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) National Quality Improvement Initiative. J Am Coll Cardiol 200545832–837. [DOI] [PubMed] [Google Scholar]
- 9.Griffith D, Hamilton K, Norrie J.et al Early and late mortality after myocardial infarction in men and women: prospective observational study. Heart 200591305–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee P Y, Alexander K P, Hammill B G.et al Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA 2001286708–713. [DOI] [PubMed] [Google Scholar]
- 11.Carrabba N, Santoro G M, Balzi D.et al In‐hospital management and outcome in women with acute myocardial infarction (data from the AMI‐Florence Registry). Am J Cardiol 2004941118–1123. [DOI] [PubMed] [Google Scholar]
- 12.Malenka D J, Wennberg D E, Quinton H A.et al Gender‐related changes in the practice and outcomes of percutaneous coronary interventions in Northern New England from 1994 to 1999. J Am Coll Cardiol 2002402092–2101. [DOI] [PubMed] [Google Scholar]
- 13.Glaser R, Herrmann H C, Murphy S A.et al Benefit of an early invasive management strategy in women with acute coronary syndromes. JAMA 20022883124–3129. [DOI] [PubMed] [Google Scholar]
- 14.Hasdai D, Porter A, Rosengren A.et al Effect of gender on outcomes of acute coronary syndromes. Am J Cardiol. 2003;91:1466–9, A6. [DOI] [PubMed]
- 15.Hannan E L, Racz M J, Arani D T.et al Short‐ and long‐term mortality for patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol 2000361194–1201. [DOI] [PubMed] [Google Scholar]
- 16.Fassa A A, Urban P, Radovanovic D.et al Trends in reperfusion therapy of ST segment elevation myocardial infarction in Switzerland: six year results from a nationwide registry. Heart 200591882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke K W, Gray D, Keating N A.et al Do women with acute myocardial infarction receive the same treatment as men? BMJ 1994309563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehilli J, Ndrepepa G, Kastrati A.et al Gender and myocardial salvage after reperfusion treatment in acute myocardial infarction. J Am Coll Cardiol 200545828–831. [DOI] [PubMed] [Google Scholar]
- 19.Mehilli J, Kastrati A, Dirschinger J.et al Sex‐based analysis of outcome in patients with acute myocardial infarction treated predominantly with percutaneous coronary intervention. JAMA 2002287210–215. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt D L, Roe M T, Peterson E D.et al Utilization of early invasive management strategies for high‐risk patients with non‐ST‐segment elevation acute coronary syndromes: results from the CRUSADE Quality Improvement Initiative. JAMA 20042922096–2104. [DOI] [PubMed] [Google Scholar]
- 21.Heer T, Gitt A K, Juenger C.et al Gender differences in acute non‐ST‐segment elevation myocardial infarction. Am J Cardiol 200698160–166. [DOI] [PubMed] [Google Scholar]
- 22.Heer T, Schiele R, Schneider S.et al Gender differences in acute myocardial infarction in the era of reperfusion (the MITRA registry). Am J Cardiol 200289511–517. [DOI] [PubMed] [Google Scholar]
- 23.Mueller C, Neumann F J, Roskamm H.et al Women do have an improved long‐term outcome after non‐ST‐elevation acute coronary syndromes treated very early and predominantly with percutaneous coronary intervention: a prospective study in 1,450 consecutive patients. J Am Coll Cardiol 200240245–250. [DOI] [PubMed] [Google Scholar]
- 24.Ben‐Ami T, Gilutz H, Porath A.et al No gender difference in the clinical management and outcome of unstable angina. Isr Med Assoc J 20057228–232. [PubMed] [Google Scholar]
- 25.Vaccarino V, Krumholz H M, Yarzebski J.et al Sex differences in 2‐year mortality after hospital discharge for myocardial infarction. Ann Intern Med 2001134173–181. [DOI] [PubMed] [Google Scholar]
- 26.Vaccarino V, Parsons L, Every N R.et al For the National Registry of Myocardial Infarction 2 Participants. Sex‐based differences in early mortality after myocardial infarction. N Engl J Med 1999341217–225. [DOI] [PubMed] [Google Scholar]
- 27.Vakili B A, Kaplan R C, Brown D L. Sex‐based differences in early mortality of patients undergoing primary angioplasty for first acute myocardial infarction. Circulation 20011043034–3038. [DOI] [PubMed] [Google Scholar]
- 28.Cantor W J, Miller J M, Hellkamp A S.et al Role of target vessel size and body surface area on outcomes after percutaneous coronary interventions in women. Am Heart J 2002144297–302. [DOI] [PubMed] [Google Scholar]
- 29.Welty F K. Cardiovascular disease and dyslipidemia in women. Arch Intern Med 2001161514–522. [DOI] [PubMed] [Google Scholar]
- 30.Clayton T C, Pocock S J, Henderson R A.et al Do men benefit more than women from an interventional strategy in patients with unstable angina or non‐ST‐elevation myocardial infarction? The impact of gender in the RITA 3 trial. Eur Heart J 2004251641–1650. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs A K, Kelsey S F, Yeh W.et al Documentation of decline in morbidity in women undergoing coronary angioplasty (a report from the 1993–94 NHLBI Percutaneous Transluminal Coronary Angioplasty Registry). National Heart, Lung, and Blood Institute. Am J Cardiol 199780979–984. [DOI] [PubMed] [Google Scholar]
- 32.Braunwald E, Antman E M, Beasley J W.et al ACC/AHA 2002 guideline update for the management of patients with unstable angina and non‐ST‐segment elevation myocardial infarction—summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina). J Am Coll Cardiol 2002401366–1374. [DOI] [PubMed] [Google Scholar]
- 33.Alter D A, Naylor C D, Austin P C.et al Biology or bias: practice patterns and long‐term outcomes for men and women with acute myocardial infarction. J Am Coll Cardiol 2002391909–1916. [DOI] [PubMed] [Google Scholar]
- 34.Hochman J S, Tamis J E, Thompson T D.et al Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med 1999341226–232. [DOI] [PubMed] [Google Scholar]
- 35.Rosengren A, Hawken S, Ounpuu S.et al Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case‐control study. Lancet 2004364953–962. [DOI] [PubMed] [Google Scholar]
- 36.Rosengren A, Wallentin L, Simoons M.et al Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur Heart J 200627789–795. [DOI] [PubMed] [Google Scholar]
- 37.Genoni M, Malacrida R, Siegrist P.et al [Therapy of acute myocardial infarct (1994–1996) at non‐university hospitals in Switzerland (CHAMI Study)]. Schweiz Med Wochenschr 19981281163–1170. [PubMed] [Google Scholar]
- 38.Andrikopoulos G K, Tzeis S E, Pipilis A G.et al Younger age potentiates post myocardial infarction survival disadvantage of women. Int J Cardiol 20051414. [DOI] [PubMed] [Google Scholar]
- 39.Simon T, Mary‐Krause M, Cambou J P.et al Impact of age and gender on in‐hospital and late mortality after acute myocardial infarction: increased early risk in younger women: results from the French nation‐wide USIC registries. Eur Heart J 2006111282–1288. [DOI] [PubMed] [Google Scholar]
- 40.Moriel M, Behar S, Tzivoni D.et al Management and outcomes of elderly women and men with acute coronary syndromes in 2000 and 2002. Arch Intern Med 20051651521–1526. [DOI] [PubMed] [Google Scholar]