Abstract
Background
The Frank–Starling law describes the relation between left ventricular volume and function. However, only a few studies have described the relation between left atrial volume (LAV) and function.
Objective
To describe an LA Frank–Starling law by studying changes in LAV measured by real‐time, three‐dimensional echocardiography (RT3DE).
Methods
LAV was calculated by RT3DE in 70 patients at end‐systole (LAVmax), end‐diastole (LAVmin) and pre‐atrial contraction (LAVpre‐A). According to LAVmax, patients were classified into three groups: LAVmax <50 ml (group I), LAVmax 50–70 ml (group II) and LAVmax >70 ml (group III). Calculated indices of LA pump function were active atrial stroke volume (SV), defined as LAVpre‐A – LAVmin, and active atrial emptying fraction (EF), defined as active atrial SV/LAVpre‐A ×100%
Results
Active atrial SV was significantly higher in group II than in group I (mean (SD) 19.0 (9.2) vs 8.2 (4.9) ml, p<0.0001), in group III it was non‐significantly lower than in group II (16.7 (12.5) vs 19.0 (9.2) ml). Active atrial SV correlated well with LAVpre‐A (r = 0.56, p<0.001), but decreased with larger LAVpre‐A. Active atrial EF tended to be higher in group II than in group I (43.1 (18.2) vs 33.2 (17.5), p<0.10), in group III it was significantly lower than in group II (26.2 (18.5) vs 43.1 (18.2), p<0.01).
Conclusion
A Frank–Starling mechanism in the left atrium could be described by RT3DE, shown by an increase in LA contractility in response to an increase in LA preload up to a point, beyond which LA contractility decreased.
Keywords: left atrial volume, left atrial function, Starling mechanism, real‐time, three‐dimensional echocardiography
The Frank–Starling law, describing the relationship between increased length of myocardial fibres and its mechanical performance, is important for cardiac function.1 The relation between myocardial preload and mechanical performance is described by a curve in which an upward position on the curve means increased performance, while a downward position means decreased myocardial performance.2 Assessment of left atrial (LA) function has important therapeutic and prognostic value.3 The instantaneous LA pressure–volume relation provides an accurate index of LA contractility.4 However, measurement of this index is invasive and technically difficult and therefore not suitable for routine clinical application.3 Non‐invasive assessment of LA contractility has been studied by two‐dimensional echocardiography, Doppler parameters, cine computed tomography, radionuclide methods and magnetic resonance imaging.5,6,7,8,9 In previous studies it was suggested that a Frank–Starling mechanism also existed in the human left atrium.10,11,12 The left atrium serves as a reservoir, conduit and booster pump for blood returning from the lungs to the heart. LA volume (LAV) is a better index of LA size,13 and owing to complex LA anatomy it may echocardiographically be best assessed by three‐dimensional echocardiography.14,15 This study aimed at describing an LA Frank–Starling law by studying changes in LAV measured by real‐time, three‐dimensional echocardiography (RT3DE).
Methods
The study comprised 70 clinically stable patients (mean age 45.6 (9.3) years, 66% men) in sinus rhythm without atrioventricular or intraventricular conduction abnormalities on a resting 12‐lead electrocardiogram. Nineteen patients (27%) were not known with cardiovascular disease, 20 patients (29%) had essential hypertension, 16 patients (23%) had coronary heart disease, and 15 patients (21%) had non‐compaction cardiomyopathy. None of these patients had mitral stenosis or significant (more than grade 1) mitral regurgitation. The patients were selected on good two‐dimensional image quality.
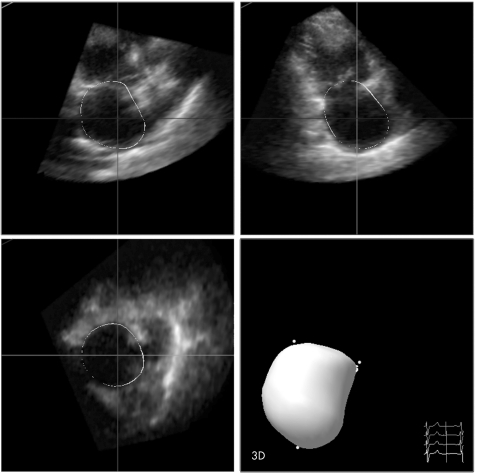
RT3DE was performed with a Sonos 7500 ultrasound system (Philips Sonos 7500, Best, The Netherlands) attached to an X4 matrix array transducer capable of providing real‐time B‐mode images. Full volume three‐dimensional images were collected within about 5–7 seconds of breath holding. Zoom function and gain adjustment were used to clarify the endocardial border. The probe position was modified to include the whole left atrium in the centre of the RT3DE image sector. The three‐dimensional dataset was transferred to a Q‐LAB system for offline analysis. Analysis of three‐dimensional images was based on a two‐dimensional approach relying on the images obtained from an apical four‐chamber view and on semi‐automated tracing of the LA endocardial border for calculation of LAV. Tracing was performed by marking five atrial points: the anterior, inferior, lateral and septal mitral annuli and the LA apex. Once this was completed, the endocardial surface was automatically delineated and the LA model could be visualised from different points of views and the LAV was obtained (fig 1). Manual modifications were made to correct automatic tracings in the majority of patients, and in particular in patients with a dilated left atrium to exclude the LA appendage and the pulmonary veins entrance from the LAV. Borders that manifested as lines were traced in the middle of the line. In addition, careful attention was given to neighbouring well‐visualised pixels as guidance for the true LA wall.
Figure 1 Quad screen display of the Q‐LAB analysis software showing methodology for left atrial volume calculation by marking the five left atrial points.
LAV was measured at three phases of the cardiac cycle: (a) maximum volume (LAVmax) obtained from an end‐systolic frame just before mitral valve opening; (b) minimum volume (LAVmin) obtained from an end‐diastolic frame just before mitral valve closure; and (c) volume before atrial contraction (LAVpre‐A) obtained from the last frame just before mitral valve reopening.
In accordance with previous studies,12,16 the following indices of LA function were assessed: (a) total atrial stroke volume (SV), defined as LAVmax − LAVmin; (b) total atrial emptying fraction (EF), defined as total atrial SV/LAVmax × 100%; (c) active atrial SV, defined as LAVpre‐A – LAVmin; (d) active atrial EF, defined as active atrial SV/LAVpre‐A ×100%; (e) passive atrial SV, defined as LAVmax − LAVpre‐A; (f) passive atrial EF as an index for LA conduit function, defined as passive atrial SV/LAVmax × 100%; and (g) atrial expansion index as an index for LA reservoir function, defined as (LAVmax − LAVmin)/LAVmin × 100%. To characterise the three phases of LA activity, passive atrial SV and EF were defined as indices for LA conduit function, active atrial SV and EF for LA pump function, and atrial expansion index for LA reservoir function.
Depending on the LAVmax values, the patients were arbitrarily classified into three groups: group I included 29 patients with LAVmax<50 ml, group II included 15 patients with LAVmax 50–70 ml and group III included 26 patients with LAVmax >70 ml.
Statistical analysis
The statistical package used was SPSS version 12.1. All LAV values and its functions were expressed as mean (SD). An independent sample t test was performed to determine whether the difference in the values was significant, with a level of significance set to p<0.05. Interobserver agreements for LAVs, were expressed according to the Bland and Altman method.17
Results
Table 1 lists the baseline criteria of the different LAV groups. There were no significant differences in age and sex distribution between the groups. Mild mitral regurgitation was present in 19 patients: 4 patients (14%) in group I, 5 patients (33%) in group II, and 10 patients (38%) in group III. All patients in groups II and III had cardiac abnormalities (hypertension, coronary artery disease, or cardiomyopathy), whereas in group I only 10 patients (34%) had cardiac abnormalities.
Table 1 Baseline criteria of the studied left atrial volume groups.
| Baseline criteria | Group I: Vmax <50 ml (n = 29) | Group II: Vmax 50–70 ml (n = 15) | Group III: Vmax >70 ml (n = 26) |
|---|---|---|---|
| Age (years), mean (SD) | 40.2 (7.5) | 44.8 (8.5) | 46.2 (9.5) |
| Male gender (%) | 17 (59) | 10 (67) | 19 (73) |
| Clinical diagnosis (%) | |||
| Normal | 19 (66) | 0 (0) | 0 (0) |
| Hypertension | 7 (24) | 10 (67) | 3 (12) |
| Coronary disease | 3 (10) | 5 (33) | 8 (31) |
| Non‐compaction CM | 0 (0) | 0 (0) | 15 (58) |
| Mitral regurgitation (%) | |||
| None | 25 (86) | 10 (67) | 16 (62) |
| Mild (grade 1) | 4 (14) | 5 (33) | 10 (38) |
CM, cardiomyopathy.
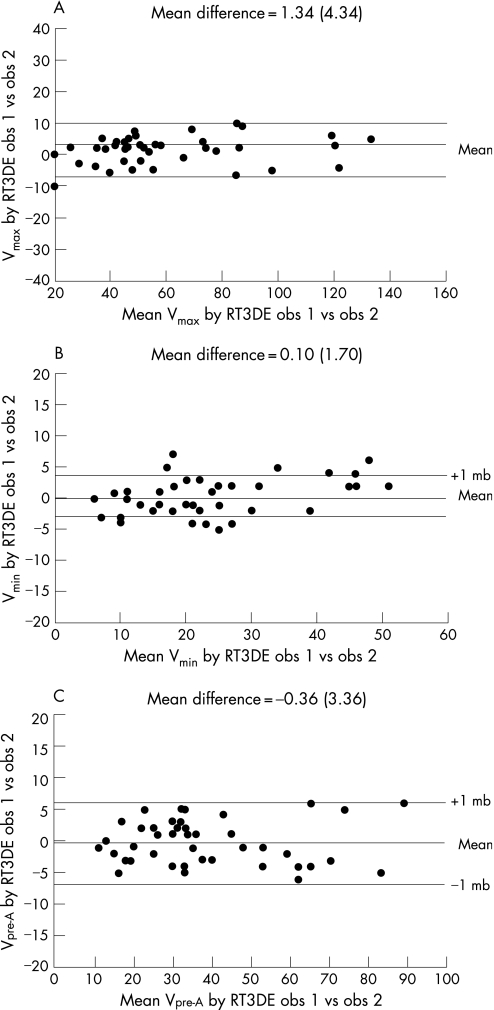
Calculation of LAV was obtained within 5–7 minutes for each patient. Absolute interobserver agreement for RT3DE was (mean difference 1.3 (4.3) ml, agreement −7.3, 10.0 ml) for LAVmax, (mean difference −0.36 (3.36) ml, agreement −7.1, 6.6 ml) for LAVpre‐A, and (mean difference −0.1 (1.7) ml, agreement −3.2, 3.6 ml) for LAVmin (fig 2).
Figure 2 Interobserver agreement for real‐time, three‐dimensional echocardiography (RT3DE) measurement of the different left atrial volumes (LAV): (A) maximum; (B) minimum and (C) pre‐atrial contraction according to the Bland and Altman principle.
LA volumes in the different patient groups
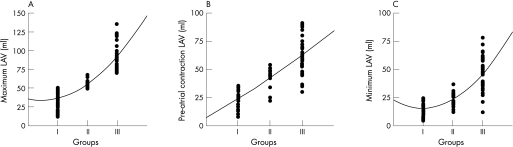
Figure 3 shows significant differences (higher values for patients with larger LAV) for LAVmax in group I compared with II (36.3 (10.7) vs 55.2 (5.7) ml, p<0.001) and in group II compared with III (55.2 (5.7) vs 92.0 (19.9) ml, p<0.001), for LAVmin in group I compared with II (15.4 (5.5) vs 23.1 (7.0) ml, p<0.001) and in group II compared with III (23.1 (7.0) vs 45.7 (15.9) ml, p<0.001), and for LAVpre‐A in group I compared with II (23.6 (7.7) vs 42.1 (9.6) ml, p<0.001) and in group II compared with III (42.1 (9.6) vs 62.4 (16.5) ml, p<0.001).
Figure 3 Left atrial volume (LAV) at three phases: (A) Maximum; (B) pre‐atrial contraction and (C) minimum in the different study groups.
LA pump function
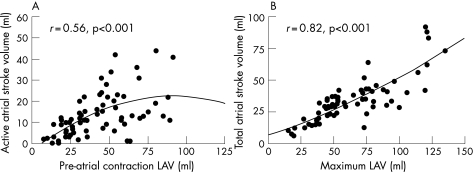
Active atrial SV was significantly higher in group II than in group I (19.0 (9.2) vs 8.2 (4.9) ml, p<0.001), in group III it was non‐significantly lower than in group II (16.7 (12.5) vs 19.0 (9.2) ml). Figure 4A shows that active atrial SV correlated well with LAVpre‐A (r = 0.56, p<0.001), but decreased with larger LAVpre‐A. Active atrial EF tended to be higher in group II than in group I (43.1 (18.2) vs 33.2 (17.5), p<0.10), in group III it was significantly lower than in group II (26.2 (18.5) vs 43.1 (18.2), p<0.01).
Figure 4 (A) Relation between pre‐atrial contraction left atrial volumes (LAV) and active atrial stroke volume; (B) maximum left atrial volumes and total atrial stroke volume.
LA conduit function
Passive atrial SV was comparable in groups I and II (12.8 (7.4) vs 13.2 (8.5) ml), but more than twofold greater in group III than in group II (29.6 (24.4) vs 13.2 (8.5) ml, p<0.005). Passive atrial EF tended to be lower in group II than in group I (23.8 (16.1) vs 34.0 (14.7)%, p<0.10), but tended to be higher in group III than in group II (30.0 (19.3) vs 23.8 (16.1)%, p<0.10).
LA reservoir function
The atrial expansion index was nearly identical in groups I and II (156.1 (97.7)% and 158.8 (78.7)%, respectively), and non‐significantly lower in group III (128.2 (107.3)%).
Total LA function
Total atrial SV was significantly larger in group II than in group I patients (32.2 (5.5) vs 20.9 (8.9) ml, p<0.001), and the largest total atrial SV was in group III (46.5 (25.5) ml, p<0.001). Figure 3B shows that total atrial SV correlated well with LAVmax (r = 0.82, p<0.001). Total atrial EF was comparable in groups I, II and III (56.4 (13.3)%, 58.5 (10.5)% and 49.9 (15.6)%, respectively).
Discussion
LA function significantly contributes to the maintenance of cardiac output, and impairment of its function contributes to circulatory failure, mitral regurgitation, atrial fibrillation and stroke.18,19 Previous studies assessed LA function by invasive pressure–volume loop determination,4 or by LAV changes assessed by nuclear scintigraphy, computed tomography or magnetic resonance imaging.7,9 RT3DE is an interestingly alternative for LAV assessment because of its availability, rapid acquisition and analysis, low cost, no need for contrast or radiation, and relatively high temporal resolution. In this study LAV was assessed in the three atrial phases by RT3DE.
To the best of our knowledge, this is the first RT3DE study to describe the existence of a Frank–Starling mechanism in the left atrium. The Frank–Starling mechanism was shown by an increase in LA contractility in response to an increase in LA preload up to a point, beyond which LA contractility decreased (fig 4A).
Despite the correlation between an increase in LAVpre‐A and active atrial SV in patients with normal to moderately enlarged LAV, active atrial SV reached a plateau and even decreased in patients with the largest LAV. These findings are in accordance with previous non‐invasive and invasive studies.3,5,20 Active atrial SV increase in response to an increase in LAVpre‐A may be related not only to a pressure increase but also to an enhanced inherent inotropic state of LA myocardium. This may explain the improvement of atrial pump function after digoxin administration in patients with heart failure.6 The clinical implication of the described Frank–Starling law in the left atrium is its role in heart failure. In early stages of heart failure, the left atrium compensates well by mechanical adaptation to the increased haemodynamic load, which may prevent or delay the appearance of symptoms of heart failure.11 Thus, evaluation of LA function in patients with heart failure will have therapeutic and prognostic value. Another clinical implication is that LA functional assessment may help as a predictor for development of atrial fibrillation and maintenance of sinus rhythm after cardioversion.21
LA conduit function is mainly determined by the rate of left ventricular relaxation.22 This may explain the tendency for a reduction in passive atrial EF in group II patients, in whom LV diastolic function is impaired owing to a high incidence of hypertension and ischaemic heart disease. The increased LA conduit function in group III patients appears as a compensatory mechanism to counterbalance decreased LA pump function.19,23 These changes in LA conduit function due to impaired left ventricular relaxation are reflected in changes in mitral inflow E/A ratio. This may explain the improvement in LA function in patients with restrictive physiology after angiotensin converting enzyme inhibitor treatment.24
LA reservoir function is determined by LA myocardial contraction and relaxation, and mitral annulus displacement during left ventricular contraction.25,26 In this study there was only a non‐significant decrease in LA reservoir function in patients with the largest LAV. This may be due to the multifactorial mechanisms responsible for LA reservoir dysfunction.
Study limitations
LA tracing can be problematic owing to (a) decreased resolution of three‐dimensional imaging compared with two‐dimensional imaging; (b) the left atrium being in the far field and (c) some LA walls suffering from lateral resolution by which pixels will become lines in the image display. Because the objective of our study was to prove a physiological concept rather than to demonstrate the feasibility of three‐dimensional assessment for LA volumes we only included patients with good image quality in our study (representing about one‐third of routinely referred patients). Because of this selection, we cannot make recommendations for the routine clinical value of LAV measurements and assessment of LAV changes. For such recommendations intra‐ and interobserver variabilities and the accuracy of such measurements (compared with a “gold standard”) should be assessed in the whole spectrum of image qualities.
Conclusion
In this RT3DE study, the presence of a Frank–Starling mechanism was shown by an increase in LA contractility in response to an increase in LA preload up to a point, beyond which LA contractility decreased. RT3DE assessment of LAV may help in understanding LA physiology and clinical assessment.
Abbreviations
EF - emptying fraction
LA - left atrial
LAV - left atrial volume
RT3DE - real‐time, three‐dimensional echocardiography
SV - stroke volume
Footnotes
Conflict of interest: None declared.
References
- 1.Fuchs F, Smith S H. Calcium, cross‐bridges, and the Frank‐Starling relationship. News Physiol Sci 2001165–10. [DOI] [PubMed] [Google Scholar]
- 2.Aurigemma G P, Zile M R, Gaasch W H. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation 2006113296–304. [DOI] [PubMed] [Google Scholar]
- 3.Nakatani S, Garcia M J, Firstenberg M S.et al Noninvasive assessment of left atrial maximum dP/dt by a combination of transmitral and pulmonary venous flow. J Am Coll Cardiol 199934795–801. [DOI] [PubMed] [Google Scholar]
- 4.Hoit B D, Shao Y, Gabel M.et al In vivo assessment of left atrial contractile performance in normal and pathological conditions using a time‐varying elastance model. Circulation 1994891829–1838. [DOI] [PubMed] [Google Scholar]
- 5.Stefanadis C, Dernellis J, Stratos C.et al Assessment of left atrial pressure‐area relation in humans by means of retrograde left atrial catheterization and echocardiographic automatic boundary detection: effects of dobutamine. J Am Coll Cardiol 199831426–436. [DOI] [PubMed] [Google Scholar]
- 6.Dernellis J M, Panaretou M P. Effects of digoxin on left atrial function in heart failure. Heart 2003891308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kircher B, Abbott J A, Pau S.et al Left atrial volume determination by biplane two‐dimensional echocardiography: validation by cine computed tomography. Am Heart J 1991121864–871. [DOI] [PubMed] [Google Scholar]
- 8.Marmor A, Frankel A, Blondeheim D S.et al Scintigraphic assessment of atrial function in patients with longstanding hypertension. Radiology 1984151483–486. [DOI] [PubMed] [Google Scholar]
- 9.Jarvinen V, Kupari M, Hekali P.et al Assessment of left atrial volumes and phasic function using cine magnetic resonance imaging in normal subjects. Am J Cardiol 1994731135–1138. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzaki M, Tamitani M, Toma Y.et al Mechanism of augmented left atrial pump function in myocardial infarction and essential hypertension evaluated by left atrial pressure‐dimension relation. Am J Cardiol 1991671121–1126. [DOI] [PubMed] [Google Scholar]
- 11.Dernellis J M, Stefanadis C I, Zacharoulis A A.et al Left atrial mechanical adaptation to long‐standing hemodynamic loads based on pressure‐volume relations. Am J Cardiol 1998811138–1143. [DOI] [PubMed] [Google Scholar]
- 12.Blondheim D S, Osipov A, Meisel S R.et al Relation of left atrial size to function as determined by transesophageal echocardiography. Am J Cardiol 200596457–463. [DOI] [PubMed] [Google Scholar]
- 13.Lester S J, Ryan E W, Schiller N B.et al Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol 199984829–832. [DOI] [PubMed] [Google Scholar]
- 14.Keller A M, Gopal A S, King D L. Left and right atrial volume by freehand three‐dimensional echocardiography: in vivo validation using magnetic resonance imaging. Eur J Echocardiogr 2000155–65. [DOI] [PubMed] [Google Scholar]
- 15.Poutanen T, Ikonen A, Vainio P.et al Left atrial volume assessed by transthoracic three dimensional echocardiography and magnetic resonance imaging: dynamic changes during the heart cycle in children. Heart 200083537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poutanen T, Jokinen E, Sairanen H.et al Left atrial and left ventricular function in healthy children and young adults assessed by three dimensional echocardiography. Heart 200389544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 19861307–310. [PubMed] [Google Scholar]
- 18.Dittrich H C, Pearce L A, Asinger R W.et al Left atrial diameter in nonvalvular atrial fibrillation: an echocardiographic study. Stroke Prevention in Atrial Fibrillation Investigators. Am Heart J 1999137494–499. [DOI] [PubMed] [Google Scholar]
- 19.Quinones M A, Greenberg B H, Kopelen H A.et al Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol 2000351237–1244. [DOI] [PubMed] [Google Scholar]
- 20.Spencer K T, Mor‐Avi V, Gorcsan J., 3rdet al Effects of aging on left atrial reservoir, conduit, and booster pump function: a multi‐institution acoustic quantification study. Heart 200185272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning W J, Silverman D I, Katz S E.et al Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol 1994231535–1540. [DOI] [PubMed] [Google Scholar]
- 22.Nikitin N P, Witte K K, Thackray S D.et al Effect of age and sex on left atrial morphology and function. Eur J Echocardiogr 2003436–42. [DOI] [PubMed] [Google Scholar]
- 23.Stefanadis C, Dernellis J, Tsiamis E.et al Effects of pacing‐induced and balloon coronary occlusion ischemia on left atrial function in patients with coronary artery disease. J Am Coll Cardiol 199933687–696. [DOI] [PubMed] [Google Scholar]
- 24.Henein M Y, Amadi A, O'Sullivan C.et al ACE inhibitors unmask incoordinate diastolic wall motion in restrictive left ventricular disease. Heart 199676326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbier P, Solomon S B, Schiller N B.et al Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 1999100427–436. [DOI] [PubMed] [Google Scholar]
- 26.Tabata T, Oki T, Yamada H.et al Role of left atrial appendage in left atrial reservoir function as evaluated by left atrial appendage clamping during cardiac surgery. Am J Cardiol 199881327–332. [DOI] [PubMed] [Google Scholar]