Abstract
Objectives
To assess the effects of intravenous magnesium on converting acute onset atrial fibrillation to sinus rhythm, reducing ventricular response and risk of bradycardia.
Design and data sources
Randomised controlled trials evaluating intravenous magnesium to treat acute onset atrial fibrillation from MEDLINE (1966 to 2006), EMBASE (1990 to 2006) and Cochrane Controlled Trials Register without language restrictions.
Review methods
Two researchers independently performed the literature search and data extraction.
Results
10 randomised controlled trials, including a total of 515 patients with acute onset atrial fibrillation, were considered. Intravenous magnesium was not effective in converting acute onset atrial fibrillation to sinus rhythm when compared to placebo or an alternative antiarrhythmic drug. When compared to placebo, adding intravenous magnesium to digoxin increased the proportion of patients with a ventricular response <100 beats/min (58.8% vs 32.6%; OR 3.2, 95% CI 1.93 to 5.42; p<0.001). When compared to calcium antagonists or amiodarone, intravenous magnesium was less effective in reducing the ventricular response (21.4% vs 58.5%; OR 0.19, 95% CI 0.09 to 0.44; p<0.001) but also less likely to induce significant bradycardia or atrioventricular block (0% vs 9.2%; OR 0.13, 95% CI 0.02 to 0.76; p = 0.02). The use of intravenous magnesium was associated with transient minor symptoms of flushing, tingling and dizziness in about 17% of the patients (OR 14.5, 95% CI 3.7 to 56.7; p<0.001).
Conclusions
Adding intravenous magnesium to digoxin reduces fast ventricular response in acute onset atrial fibrillation. The effect of intravenous magnesium on the ventricular rate and its cardiovascular side effects are less significant than other calcium antagonists or amiodarone. Intravenous magnesium can be considered as a safe adjunct to digoxin in controlling the ventricular response in atrial fibrillation.
Atrial fibrillation is the commonest cardiac arrhythmia in clinical practice. Atrial fibrillation affects an estimated 2.2 million adults in the USA and has an estimated incidence of 1.0 per 1000 person‐years in the UK.1,2 Atrial fibrillation is associated with significant morbidity and mortality. Patients in atrial fibrillation have a fivefold increased risk of thromboembolic stroke and twofold increased risk of death when compared to the general population.3,4 Atrial fibrillation can also cause tachycardia‐induced heart failure if the rapid ventricular response is sustained for a prolonged period of time.5
Most patients in acute atrial fibrillation have no significant haemodynamic instability and as such, pharmacological therapy is usually the initial treatment of choice. A variety of pharmacological agents can be used, either to control the rapid ventricular response or convert the arrhythmia to sinus rhythm, with variable results. The agents evaluated include digoxin, beta‐blockers, calcium antagonists, flecainide, propafenone, ibutilide, and amiodarone.6 However, in patients with impaired left ventricular function, digoxin or amiodarone is the pharmacological agent of choice because of their minimal negative inotropic effects.6
Magnesium has many significant physiological and pharmacological effects on different organ systems. The mechanisms of its action include calcium antagonism, regulation of energy transfer and membrane stabilisation.7 Intravenous magnesium has a high therapeutic‐to‐toxic ratio and minimal negative inotropic effects.8,9 Intravenous magnesium can reduce automaticity,10 atrioventricular nodal conduction,11,12 polymorphic ventricular tachycardia due to prolonged QT interval and digoxin‐induced arrhythmias.7,8,13 Prophylactic use of intravenous magnesium can also reduce the occurrence of atrial fibrillation after cardiac surgery.14 However, there are no large randomised controlled studies or meta‐analyses that evaluate intravenous magnesium as an antiarrhythmic agent in the setting of acute onset atrial fibrillation.
Rhythm control by pharmacological agents is often most effective when the drug is initiated within 10 days of onset of atrial fibrillation.15 We hypothesised that intravenous magnesium could be an effective antiarrhythmic agent in patients with acute onset atrial fibrillation. We assessed the potential beneficial and harmful effects of intravenous magnesium, when compared to placebo or an alternative arrhythmic agent, in the setting of acute onset atrial fibrillation (<7 days) in this meta‐analysis. The end‐points assessed in this study included rhythm control, ventricular response <100 beats/minute, bradycardia, hypotension, and other side effects.
Methods
Search strategy
Two researchers searched the Cochrane Controlled Trials Register (2006 issue 1), EMBASE (January 1990 to May 2006) and MEDLINE databases (1966 to May 2006) independently. During the electronic database search, the following exploded MeSH terms were used: “magnesium”, with “atrial fibrillation”, “supraventricular arrhythmia” or “supraventricular tachyarrhythmia”. The search was limited to clinical trials, letters, editorials, reviews or randomised controlled trials. The reference lists of related editorials, reviews and original articles identified were searched for relevant trials. Finally, the websites of the International Network of Agencies of Health Technology Assessment and International Society of Technology Assessment in Health Care were searched to ensure all suitable trials were included. There were no language restrictions for inclusion in this meta‐analysis.
Selection criteria and validity assessment
Only randomised controlled clinical trials comparing intravenous magnesium with placebo or an alternative antiarrhythmic drug to treat acute onset atrial fibrillation in adult patients were included. In this meta‐analysis, acute onset atrial fibrillation was defined as onset of symptoms or electrocardiography documented atrial fibrillation of less than 7 days before trial enrolment. In trials that included patients with atrial fibrillation, atrial flutter or other supraventricular arrhythmia, only the subset of patients with atrial fibrillation was included if the data were available. Trials that assessed chronic atrial fibrillation or evaluated oral magnesium were excluded. Furthermore, trials that evaluated the prophylactic use of magnesium to prevent rather than to treat atrial fibrillation were also excluded.
Two independent reviewers examined the full text of all identified trials to confirm they fulfilled the inclusion criteria. They examined and recorded the trial characteristics and outcomes independently, using a predesigned data abstraction form. This abstraction form was used to record information regarding the quality of the trial such as allocation concealment, randomisation method, blinding of treatment, and inclusion and exclusion criteria. The quality of the trial was scored according to the Jadad scale (range from 0 to 5, with the higher scales indicating a better quality trial)16 but the individual component that constitutes the quality of the trial was also described. The grading of allocation concealment was based on the Cochrane approach, that is, adequate or uncertain or clearly inadequate. A third independent researcher checked the completed data abstract forms and there were no disagreements between the three independent reviewers in the data extracted. We wrote to the leading author of an included trial to clarify the data of the trial and received additional information about the trial.17 Data were checked and entered into the Review Manager (version 4.2.6 for Windows. Oxford, England: The Cochrane Collaboration, 2003) database for further analyses.
Outcomes of interest
The proportion of patients with atrial fibrillation converted to sinus rhythm within 24 hours of treatment and the proportion of patients that ventricular response slowed to less than 100 beats/minute were chosen as the main outcomes because they are the most relevant clinical outcomes in patients with acute onset atrial fibrillation. There were no missing data for these two main outcomes in the trials included. The other outcomes assessed included the proportion of patients who developed symptoms of flushing, tingling and dizziness, the proportion of patients with significant bradycardia or atrioventricular block (pause >3 seconds, hypotension or symptomatic), hypotension (systolic blood pressure <100 mm Hg or symptomatic), and also the proportion of patients who required rescue antiarrhythmic drugs at the end of the trial.
Statistical analysis
The differences in categorical outcomes between magnesium and placebo or an alternative antiarrhythmic drug were reported as odds ratio (OR) with 95% confidence interval (CI), using a random effect model. The trials were further stratified into trials that compared magnesium with placebo and trials that compared magnesium with another antiarrhythmic agent, and the interaction between the two strata of trials was tested by ratio of odds ratio.18 The presence of heterogeneity between trials was assessed by the χ2 statistics and the extent of inconsistency was assessed using I2 statistics.19 One trial reported that several patients had flushing, tingling and dizziness after intravenous magnesium treatment but did not specify the exact number of patients and so this trial was excluded from the analysis.28 Sensitivity analyses were conducted by excluding trials that included some patients with atrial flutter or other supraventricular tachyarrhythmias17,20,21,22,27 and trials that included patients with undocumented duration of atrial fibrillation.20,22,23 Publication bias was assessed by funnel plot using conversion to sinus rhythm as an end point. All tests were two‐tailed and a p‐value less than 0.05 was regarded as significant in this meta‐analysis.
Results
Study selection and description
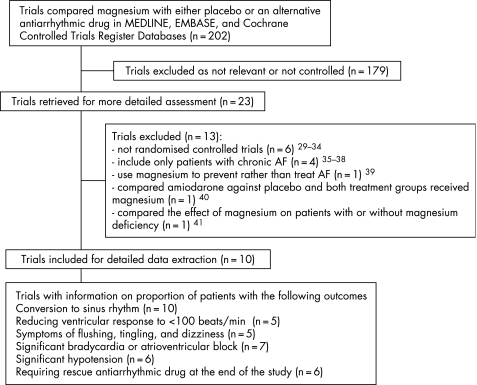
Our electronic searches identified 202 studies of which 10 fulfilled the inclusion criteria17,20,21,22,23,24,25,26,27,28 and were subjected to meta‐analysis (fig 1). There was complete agreement on inclusion assessment between the three reviewers. The 10 included trials involved data from six countries and all were published in English (table 1). Three trials involved patients in the emergency department,21,23,24 three trials involved patients in the intensive care unit,17,20,27 and three trials involved patients in the cardiology department or ward.22,25,26 One trial did not specify the hospital location of the patients in the trial.28 Five trials compared magnesium with placebo.21,22,23,24,25 Among these five trials, four of them used digoxin 21,23,24,25 and one trial used ibutilide as the concurrent antiarrhythmic drug with both placebo and magnesium.22 In one trial that compared magnesium with placebo, the first part of the trial compared magnesium with placebo only and the second part of the trial tested the effect of adding digoxin to both the magnesium and placebo group. The data of the second part of this trial were pooled separately from the first part of the trial in this meta‐analysis.21 The other five trials compared magnesium with another antiarrhythmic drug without the use of placebo.17,20,26,27,28 Among the five trials that compared magnesium with another antiarrhythmic drug, three of them compared magnesium with intravenous calcium antagonists 20,26,28 and two trials compared magnesium with amiodarone.17,27 All trials except one explicitly stated that they excluded patients who had unstable blood pressure (systolic blood pressure <80–90 mm Hg) and renal dysfunction.
Figure 1 Flow chart showing trial inclusion and exclusion in this meta‐analysis.
Table 1 Characteristics of the included trials.
| Study, year, country of origin | Participants, inclusion and exclusion criteria | Interventions | Outcomes and observed period | Allocation concealment, blinding, % loss to follow‐up, intention to treat analysis, and Jadad's scale (0–5) |
|---|---|---|---|---|
| Kiziltepe et al., 2003, Turkey17 | 20 adult patients after cardiac surgery in ICU with any supraventricular tachyarrhythmias (60% with AF & the duration before treatment <1 week). Exclusion: arrhythmias before surgery, hypokalaemia, hypotension, receiving antiarrhythmics or beta‐blockers | Amiodarone group (n = 10): 150 mg +60 mg/h for 6 h then 30 mg/h for 18 h. | Rhythm conversion, bradycardia or atrioventricular block, hypotension, requiring rescue antiarrhythmics. Observed for 2 h | Allocation concealment unclear, unblinded, all patients completed the study, analysis by intention to treat. 2 |
| Magnesium group (n = 10): 1.5 g over 5–10 min and repeat if no response in 15 min | ||||
| Joshi et al., 1995, India20 | 86 patients in ICU with AF or atrial flutter >160/min (the proportion of AF and flutter & the exact duration of AF before treatment were not reported). | Verapamil group (n = 45): 5 mg intravenous verapamil and repeat if no response after 15 min. | Rhythm conversion, ventricular response rate <100/min, bradycardia or hypotension. Observed for 20 min | Allocation concealment unclear, unblinded, all patients completed the trial, analysis by intention to treat. 2 |
| Exclusion: renal dysfunction, hypotension | Magnesium group (n = 41): 2.0 g and repeat if no response after 15 min | |||
| Walker et al., 1996, Australia21 | 42 patients with either AF (90.2%) or atrial flutter (9.8%) >100/min duration <48 h, at ED. | Controlled group (n = 20): Plain 20 ml normal saline, intravenous digoxin at the discretion of the treating physician after first 30 min (86% at the end). | Rhythm conversion, ventricular response rate <100/min, bradycardia or atrioventricular block, flushing, tingling, dizziness, requiring rescue antiarrhythmics. Observed for 30 min initially followed by a second period of 6 h | Allocation concealment unclear, double‐blinded, 2.3% not able to complete the trial, not by intention to treat. 4 |
| Exclusion: hypotension, renal dysfunction, drug overdose, receiving antiarrhythmic drug other than digoxin | Magnesium group (n = 22): 5 g magnesium in 20 ml normal saline, intravenous digoxin at the discretion of the treating physician after first 30 min (70% at the end) | |||
| Caron et al., 2003, USA22 | 20 patients in cardiology department with AF (60%) or flutter (40%) before intravenous ibutilide treatment (minimal rate and duration not defined). | Controlled group (n = 9): 50 ml normal saline10 min before intravenous ibutilide (1 mg). | Rhythm conversion. Observed for 60 min | Allocation concealment unclear, double‐ blinded, all patients completed the trial, analysis by intention to treat. 4 |
| Exclusion: renal dysfunction, magnesium treatment within 8 h, pregnant, receiving antiarrhythmics | Magnesium group (n = 11): 2 g magnesium over 10 min then 2 g over 1 h, intravenous ibutilide after first 2 g magnesium | |||
| Hays et al., 1994, USA23 | 15 patients with AF >99/min, for a mean duration of 4 to 6 days at ED. | Controlled group (n = 8): placebo (not defined), intravenous 0.5 mg digoxin after first 30 min. | Rhythm conversion, hypotension, flushing, tingling, dizziness. Observed for 4 h | Allocation concealment unclear, double‐blinded, all patients completed the trial, analysis by intention to treat. 3 |
| Exclusion: receiving antiarrhythmics, digoxin, beta‐blockers, calcium antagonists, hypokalaemia, atrioventricular block, hypotension | Magnesium group (n = 7): 2 g over 1 min, then 1 g/h for 4 h, intravenous 0.5 mg digoxin after first 30 min | |||
| Davey et al., 2005, Australia24 | 199 patients with AF >120/min, 62% <24 h in duration, at ED. | Controlled group (n = 97): 100 ml 5% dextrose, intravenous digoxin at the discretion of treating physician (86.2% at the end). | Rhythm conversion, ventricular response rate <100/min, bradycardia or atrioventricular block, flushing, tingling, dizziness, requiring rescue antiarrhythmics. Observed for 150 min | Adequate allocation concealment, triple‐blinded, 8.5% could not complete the trial, analysis by intention to treat. 5 |
| Exclusion: hypotension, renal dysfunction, atrioventricular block, myocardial infarction | Magnesium group (n = 102): 2.5 g over 20 min then 2.5 g over 2 h, intravenous digoxin at the discretion of treating physician (77.6% at the end) | |||
| Brodsky et al., 1994, USA25 | 18 cardiology outpatients with AF >100/min & <7 days in duration. | Controlled group (n = 8): 100 ml 5% dextrose + simultaneous intravenous digoxin 0.375 to 0.625 mg (adjusted with body weight and then up to 3 more doses of digoxin from 0.125 to 0.375 mg). | Rhythm conversion, ventricular response rate <90/min, bradycardia or atrioventricular block. Observed for 24 h | Allocation concealment unclear, double‐blinded, all patients completed the trial, analysis by intention to treat. 4 |
| Exclusion: hypotension, renal dysfunction, receiving antiarrhythmics, digoxin blood concentration >0.8 nmol/l | Magnesium group (n = 10): 2 g in 100 ml 5% dextrose over 15 min then 8 g over 6 h. | |||
| Chiladakis et al., 2001, Greece26 | 46 patients with AF >100/min & <12 h in duration in cardiology department. | Diltiazem group (n = 23): intravenous diltiazem 25 mg over 15 min, then 12.5 mg/h infusion for 6 h. | Rhythm conversion, bradycardia or atrioventricular block, hypotension, flushing, tingling, dizziness, requiring rescue antiarrhythmics. Observed for 6 h | Allocation concealment unclear, single‐blinded, all patients completed the trial, analysis by intention to treat. 2 |
| Exclusion: myocardial infarction, hypotension, atrioventricular block, sick sinus syndrome | Magnesium group (n = 23): 2.5 g over 15 min, then 7.5 g over 6 h | |||
| Moran et al., 1995, Australia27 | 34 ICU patients with AF >120/min, duration 1 h to <6 months. | Amiodarone group (n = 18): intravenous amiodarone 5 mg/kg over 15–20 min, then 10 mg/kg over 24 h. | Rhythm conversion, requiring rescue antiarrhythmics. Observed for 24 h | Adequate allocation concealment, unblinded, 4.8% could not complete the trial, analysis by intention to treat. 3 |
| Exclusion: hypotension, renal dysfunction if not on dialysis, serum potassium concentration <4.0 mmol/l | Magnesium group (n = 16): 0.15 mmol (0.6 g)/kg/h over 5 min, then 0.1 mmol (0.4 g)/kg/hr for 24 h | |||
| Gullestad et al., 1993, Norway28 | 35 hospitalised patients (exact location not defined) with AF >100/min & <1 week in duration. | Verapamil group (n = 20): intravenous verapamil 5 mg over 5 min, repeat 5 mg if no response after 10 min, followed by 0.1 mg/min infusion. | Rhythm conversion, bradycardia ventricular response rate <90/min, or atrioventricular block, hypotension, flushing, tingling, dizziness, requiring rescue antiarrhythmics. Observed for 4 h | Adequate allocation concealment, single‐blinded, 5.7% could not complete the trial, analysis by intention to treat. 3 |
| Exclusion: severe congestive heart failure, hypotension, atrioventricular block, sick sinus syndrome, renal dysfunction, myocardial infarction. | Magnesium group (n = 15): 1.2 g over 5 min, repeat if no response after 10 min, followed by infusion 0.04 mmol (0.01 g)/min |
AF, atrial fibrillation; ICU, intensive care unit.
The baseline mean serum magnesium concentrations in both treatment arms were reported to be normal in six trials but this information was not reported in the other four trials.17,21,22,26 The doses of intravenous magnesium used ranged between 12 and 40 mmol (3–10 g) in all trials except one which used a prolonged magnesium infusion over 24 hours and the total dose of magnesium was estimated to be 100 mmol (25 g) for a 80 kg patient.27 The period of time assessed for the selected clinical end points after initiation of magnesium treatment ranged between 20 minutes and 24 hours (mean 7 hours, median 4 hours).
Assessment of validity
The quality of the included trials varied; the Jadad scale ranged from 2 to 5 (mean 3.2). All trials were randomised but only five trials were double‐blinded.21,22,23,24,25 All the five trials that compared magnesium with an alternative antiarrhythmic drug were not double‐blinded. All the trials except one were analysed by the intention to treat principle and the proportion of patients lost or excluded was less than 10% in all the included trials. The agreement between reviewers was over 90% for different criteria.
Effect of intravenous magnesium on rhythm conversion, ventricular response rate, and cardiovascular and systemic side effects
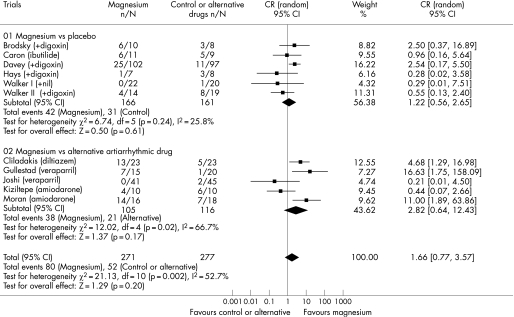
Ten trials involving 515 patients reported data on the effect of intravenous magnesium on rhythm conversion. Adding intravenous magnesium to either digoxin or ibutilide did not increase the rate of converting atrial fibrillation to sinus rhythm (25.3% vs 19.3%; odds ratio [OR] 1.22, 95% confidence interval [CI] 0.56 to 2.65; p = 0.61). Intravenous magnesium alone was also no better than calcium antagonists or amiodarone in achieving sinus rhythm in patients with acute onset atrial fibrillation (36.2% vs 18.2%; OR 2.82, 95% CI 0.64 to 12.43; p = 0.17) (fig 2). However, there was significant heterogeneity in the comparison between magnesium and an alternative antiarrhythmic drug (I2 = 66.7%, χ2 test for heterogeneity = 0.02), likely to be due to different doses of magnesium and different antiarrhythmic drugs used in the pooled trials.
Figure 2 Forest plot showing the effect of intravenous magnesium on conversion of atrial fibrillation to sinus rhythm as compared to placebo or an alternative antiarrhythmic drug.
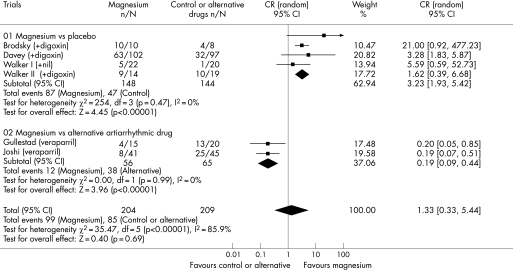
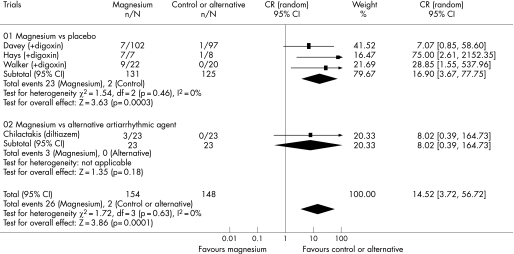
Five trials involving 380 patients reported data on the effect of intravenous magnesium on ventricular response rate. Adding intravenous magnesium to digoxin increased the proportion of patients with a ventricular response rate <100 beats/min (58.8% vs 32.6%; OR 3.23, 95% CI 1.93 to 5.42; p<0.001). However, when intravenous magnesium was compared with intravenous verapamil, magnesium was less effective than intravenous verapamil in controlling the ventricular response rate (21.4% vs 58.5%; OR 0.19, 95% CI 0.09 to 0.44; p<0.001) (fig 3). There was a significant difference in the comparisons between the two strata of trials (ratio of OR 16.9, 95% CI 6.6 to 43.4; p<0.001), suggesting that verapamil was better than placebo in controlling the ventricular response rate.
Figure 3 Forest plot showing the effect of intravenous magnesium on reducing ventricular response rate to less than 100 beats/min when compared to placebo or an alternative antiarrhythmic drug.
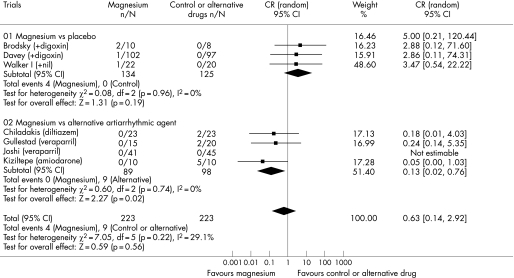
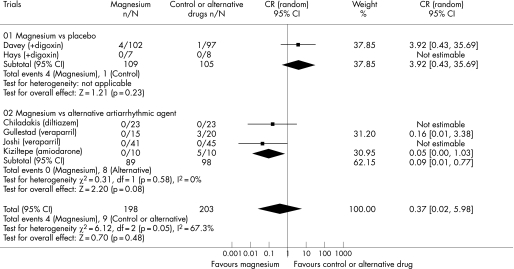
Seven trials involving 446 patients reported data on the risk of significant bradycardia or atrioventricular block. Intravenous magnesium was less likely to induce bradycardia or atrioventricular block (0% vs 9.2%; OR 0.13, 95% CI 0.02 to 0.76; p = 0.02) and hypotension (0% vs 8.2%; OR 0.09, 95% CI 0.01 to 0.77; p = 0.03) when compared to intravenous calcium antagonists or amiodarone (fig 4, 5). Adding magnesium to digoxin was not associated with a significant risk of bradycardia or atrioventricular block (3% vs 0%; OR 3.47, 95% CI 0.54 to 22.22; p = 0.19) and hypotension (3.7% vs 1.0%; OR 3.92, 95% CI 0.43 to 35.69; p = 0.23). There was a significant difference in the comparisons between two strata of trials in both cardiovascular side effects suggesting that calcium antagonists or amiodarone were more likely than placebo to cause bradycardia or atrioventricular block (ratio of OR 26.7, 95% CI 2.0 to 361.4; p = 0.01) and hypotension (ratio of OR 44, 95% CI 2.0 to 972.0; p = 0.02). The use of intravenous magnesium was associated with transient minor symptoms of flushing, tingling and dizziness in about 17% of the patients (OR 14.5, 95% CI 3.7 to 56.7; p<0.001) (fig 6). There was no significant difference in the proportion of patients requiring rescue antiarrhythmic drug when magnesium was compared to placebo or an alternative antiarrhythmic drug (overall OR 0.84, 95% CI 0.25 to 2.81). A cost analysis was reported in two trials25,27 but none reported a formal cost‐effectiveness analysis.
Figure 4 Forest plot showing the effect of intravenous magnesium on the risk of bradycardia or atrioventricular block when compared to placebo or an alternative antiarrhythmic drug.
Figure 5 Forest plot showing the effect of intravenous magnesium on the risk of hypotension when compared to placebo or an alternative antiarrhythmic drug.
Figure 6 Forest plot showing the effect of intravenous magnesium on the risk of symptoms of flushing, tingling and dizziness.
Sensitivity analyses and publication bias
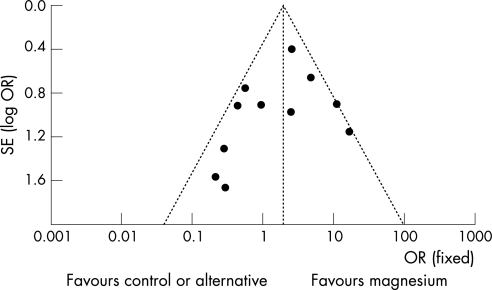
We could not completely identify data of the patients with atrial fibrillation only in five studies. Excluding these trials that included a small proportion of patients with atrial flutter or other supraventricular tachyarrhythmias17,20,21,22,27 (p = 0.21) or three trials that included patients with undocumented duration of atrial fibrillation20,22,23 (p = 0.44) did not change the effect of magnesium on rhythm control when it was compared to placebo. However, excluding trials that included some patients with atrial flutter or other supraventricular tachyarrhythmias17,20,21,22,27 showed that intravenous calcium antagonists were more effective than magnesium in converting atrial fibrillation to sinus rhythm (OR 6.4, 95% CI 2.1 to 19.6; p = 0.001). A funnel plot showed a slight asymmetry in the distribution of the mean estimates of the trials. This could be due to the small number of trials included in this meta‐analysis42 or a small publication bias that favoured the publication of trials that showed an alternative antiarrhythmic drug was better than intravenous magnesium on rhythm control (fig 7).
Figure 7 Funnel plot using rhythm control as an end point shows the possibility of a small publication bias.
Discussion
This meta‐analysis shows that adding intravenous magnesium is more effective than placebo in reducing the fast ventricular response rate when added to digoxin but not in achieving sinus rhythm in patients with acute atrial fibrillation. With the limited data available, the effects of intravenous magnesium on the ventricular response rate and the risk of bradycardia, atrioventricular block or hypotension are less significant than intravenous calcium antagonists or amiodarone. Minor transient symptoms such as flushing, tingling and dizziness are not uncommon (17%) after the use of intravenous magnesium.
While previous meta‐analysis has shown that magnesium is effective in preventing the occurrence of atrial fibrillation after cardiac surgery,14 our study shows that adding intravenous magnesium to either digoxin or ibutilide is not effective in achieving sinus rhythm once atrial fibrillation has occurred. The difference in our results and the previous meta‐analysis that assessed prophylactic use of magnesium could be due to different underlying causes of atrial fibrillation and different sample size. Magnesium deficiency is common after cardiac surgery7,17 and there is a suggestion that patients who have both hypomagnesaemia and atrial fibrillation are more likely to respond to intravenous magnesium.17,41 Six of the 10 trials in this meta‐analysis included patients with a normal baseline serum magnesium concentration before magnesium treatment. This factor could have selected the group of patients that were less likely to respond to intravenous magnesium in achieving sinus rhythm. Furthermore, the sample size of this meta‐analysis (n = 515) is still relatively small and could only show a significant effect on rhythm control if intravenous magnesium can increase the conversion rate from 20% to at least 30%. Nevertheless, the average rhythm conversion rate in the magnesium group of this meta‐analysis was only 25% and this was still far below the 60% spontaneous rhythm conversion rate of acute onset atrial fibrillation reported in the literature.43,44 Similarly, we could not exclude a small increase in risk of hypotension and bradycardia with intravenous magnesium when compared to placebo because of the relatively small number of patients included in the trials.
Our results show that the therapeutic effect of intravenous magnesium is mainly on reducing the fast ventricular response rate in patients with acute onset atrial fibrillation. Its effectiveness and also the associated cardiovascular side effects are less significant than intravenous calcium antagonists or amiodarone. Magnesium has many physiological effects and one of the possible mechanisms for its action may include the calcium channel blockade effect on the atrioventricular node.7,11,12 If this is the main mechanism how intravenous magnesium works in patients with atrial fibrillation, then our results suggest that intravenous magnesium can be regarded as a “weak” intravenous calcium antagonist in both effectiveness (ie, slowing the ventricular rate) and toxicity (ie, bradycardia and hypotension) when compared to verapamil and diltiazem. However, minor transient side effects including flushing, tingling and dizziness are not uncommon and appear to be specifically associated with magnesium but not other calcium antagonists. Because magnesium has multiple pharmacological actions other than calcium antagonism on the atrioventricular node, some of these minor side effects could be due to the other actions of magnesium including its peripheral vasodilating effect and antagonistic action on the N‐methyl D‐aspartate receptor.7 It should also be noted that magnesium can accumulate in patients with renal failure and a very high serum magnesium concentration (>5.0 mmol/l) can cause neuromuscular blockade and respiratory depression, although these side effects were not reported in the pooled studies.7
There are some limitations with this study. First, although serum magnesium concentrations were normal and comparable in both treatment groups in six of the included trials, this did not exclude subclinical imbalance in magnesium deficiency between the two treatment groups because of the poor correlation between serum and myocyte magnesium concentrations.45 This could represent a potential confounder that we could not exclude with the data of the trials. Second, this study included trials that evaluated different doses of intravenous magnesium and also compared magnesium to different alternative antiarrhythmic drugs in different patient cohorts. The results of the comparison between intravenous magnesium and placebo were homogenous but there was significant heterogeneity in the comparison between intravenous magnesium and another antiarrhythmic drug. This was likely due to different doses of magnesium and different types of antiarrhythmic drugs used in this stratum of trials. Third, multiple statistical testing was performed in the comparison between magnesium and placebo because one trial was analysed twice.21 However, using the Bonferroni correction, the significance of the results in assessing the effects of magnesium on rhythm control and ventricular rate <100 beats/min remains unchanged.
In conclusion, intravenous magnesium, when compared to other antiarrhythmic agents or with digoxin, is not effective in converting acute onset atrial fibrillation to sinus rhythm in patients with a normal serum magnesium concentration. Adding intravenous magnesium to digoxin reduces the fast ventricular response but this effect and its associated cardiovascular side effects are both less significant than other calcium antagonists or amiodarone. Intravenous magnesium can be considered as a safe adjunct to digoxin in controlling the ventricular response in patients with acute onset atrial fibrillation. A large randomised controlled trial is needed to confirm our findings.
Acknowledgements
We thank the Australasian College of Emergency Physicians for providing a reprint of one of the trials included in this meta‐analysis and Dr Ugursay Kiziltepe for providing details of his original study.
Footnotes
Funding: No financial support was received for this study from pharmaceutical companies or other private companies in the form of grants and awards. This study was solely funded by the Department of Intensive Care, Royal Perth Hospital.
Competing interests: None.
References
- 1.Feinberg W M, Blackshear J L, Laupacis A.et al Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 1995155469–473. [PubMed] [Google Scholar]
- 2.Ruigomez A, Johansson S, Wallander M A.et al Predictors and prognosis of paroxysmal atrial fibrillation in general practice in the UK. BMC Cardiovasc Disord 2005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel W B, Wolf P A, Benjamin E J.et al Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population‐based estimates. Am J Cardiol 1998822N–9N. [DOI] [PubMed] [Google Scholar]
- 4.Ruigomez A, Johansson S, Wallander M A.et al Risk of mortality in a cohort of patients newly diagnosed with chronic atrial fibrillation. BMC Cardiovasc Disord 200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nerheim P, Birger‐Botkin S, Piracha L.et al Heart failure and sudden death in patients with tachycardia‐induced cardiomyopathy and recurrent tachycardia. Circulation 2004110247–252. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal M B, Taneja A K, Lip G Y, Flather M. Recent developments in atrial fibrillation. BMJ 2005330238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fawcett W J, Haxby E J, Male D A. Magnesium: physiology and pharmacology. Br J Anaesth 199983302–320. [DOI] [PubMed] [Google Scholar]
- 8.Crippa G, Sverzellati E, Giorgi‐Pierfranceschi M.et al Magnesium and cardiovascular drugs: interactions and therapeutic role. Ann Ital Med Int 19991440–45. [PubMed] [Google Scholar]
- 9.Rasmussen H S, Larsen O G, Meier K.et al Hemodynamic effects of intravenously administered magnesium on patients with ischemic heart disease. Clin Cardiol 198811824–828. [DOI] [PubMed] [Google Scholar]
- 10.Iseri L T, Allen B J, Ginkel M L.et al Ionic biology and ionic medicine in cardiac arrhythmias with particular reference to magnesium. Am Heart J 19921231404–1409. [DOI] [PubMed] [Google Scholar]
- 11.DiCarlo L A, Jr, Morady F, de Buitleir M.et al Effects of magnesium sulfate on cardiac conduction and refractoriness in humans. J Am Coll Cardiol 198671356–1362. [DOI] [PubMed] [Google Scholar]
- 12.Christiansen E H, Frost L, Andreasen F.et al Dose‐related cardiac electrophysiological effects of intravenous magnesium. A double‐blind placebo‐controlled dose‐response study in patients with paroxysmal supraventricular tachycardia. Europace 20002320–326. [DOI] [PubMed] [Google Scholar]
- 13.Khan I A, Gowda R M. Novel therapeutics for treatment of long‐QT syndrome and torsade de pointes. Int J Cardiol 2004951–6. [DOI] [PubMed] [Google Scholar]
- 14.Miller S, Crystal E, Garfinkle M.et al Effects of magnesium on atrial fibrillation after cardiac surgery: a meta‐analysis. Heart 200591618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borgeat A, Goy J J, Maendly R.et al Flecainide versus quinidine for conversion of atrial fibrillation to sinus rhythm. Am J Cardiol 198658496–498. [DOI] [PubMed] [Google Scholar]
- 16.Jadad A R, Moore R A, Carroll D.et al Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996171–12. [DOI] [PubMed] [Google Scholar]
- 17.Kiziltepe U, Eyileten Z B, Sirlak M.et al Antiarrhythmic effect of magnesium sulfate after open heart surgery: effect of blood levels. Int J Cardiol 200389153–158. [DOI] [PubMed] [Google Scholar]
- 18.Altman D G, Bland J M. Interaction revisited: the difference between two estimates. BMJ 2003326219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J P, Thompson S G, Deeks J J.et al Measuring inconsistency in meta‐analyses. BMJ 2003327557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi P P, Deshmukh P K, Salkar R G. Efficacy of intravenous magnesium sulphate in supraventricular tachyarrhythmias. J Assoc Physicians India 199543529–531. [PubMed] [Google Scholar]
- 21.Walker S, Taylor J, Harrod R. The acute effects of magnesium in atrial fibrillation and flutter with a rapid ventricular rate. Emerg Med 19968207–213. [Google Scholar]
- 22.Caron M F, Kluger J, Tsikouris J P.et al Effects of intravenous magnesium sulfate on the QT interval in patients receiving ibutilide. Pharmacotherapy 200323296–300. [DOI] [PubMed] [Google Scholar]
- 23.Hays J V, Gilman J K, Rubal B J. Effect of magnesium sulfate on ventricular rate control in atrial fibrillation. Ann Emerg Med 19942461–64. [DOI] [PubMed] [Google Scholar]
- 24.Davey M J, Teubner D. A randomized controlled trial of magnesium sulfate, in addition to usual care, for rate control in atrial fibrillation. Ann Emerg Med 200545347–353. [DOI] [PubMed] [Google Scholar]
- 25.Brodsky M A, Orlov M V, Capparelli E V.et al Magnesium therapy in new‐onset atrial fibrillation. Am J Cardiol 1994731227–1229. [DOI] [PubMed] [Google Scholar]
- 26.Chiladakis J A, Stathopoulos C, Davlouros P.et al Intravenous magnesium sulfate versus diltiazem in paroxysmal atrial fibrillation. Int J Cardiol 200179287–291. [DOI] [PubMed] [Google Scholar]
- 27.Moran J L, Gallagher J, Peake S L.et al Parenteral magnesium sulfate versus amiodarone in the therapy of atrial tachyarrhythmias: a prospective, randomized study. Crit Care Med 1995231816–1824. [DOI] [PubMed] [Google Scholar]
- 28.Gullestad L, Birkeland K, Molstad P.et al The effect of magnesium versus verapamil on supraventricular arrhythmias. Clin Cardiol 199316429–434. [DOI] [PubMed] [Google Scholar]
- 29.Eray O, Akca S, Pekdemir M.et al Magnesium efficacy in magnesium deficient and nondeficient patients with rapid ventricular response atrial fibrillation. Eur J Emerg Med 20007287–290. [DOI] [PubMed] [Google Scholar]
- 30.Kalus J S, Spencer A P, Tsikouris J P.et al Impact of prophylactic i.v. magnesium on the efficacy of ibutilide for conversion of atrial fibrillation or flutter. Am J Health Syst Pharm 2003602308–2312. [DOI] [PubMed] [Google Scholar]
- 31.Henyan N, White C M. Adjunctive intravenous magnesium to reduce toxicity and enhance efficacy of class III antiarrhythmic agents. Conn Med 200468627–629. [PubMed] [Google Scholar]
- 32.Floriot C, Delacour J L, Bourscheid D.et al Value of magnesium chloride in supraventricular paroxysmal tachycardia and tachycardia caused by auricular fibrillation. Presse Med 198716829. [PubMed] [Google Scholar]
- 33.Coleman C I, Kalus J S, White C M.et al Cost effectiveness of ibutilide with prophylactic magnesium in the treatment of atrial fibrillation. Pharmacoeconomics 200422877–883. [DOI] [PubMed] [Google Scholar]
- 34.Wesley R C, Jr, Haines D E, Lerman B B.et al Effect of intravenous magnesium sulfate on supraventricular tachycardia. Am J Cardiol 1989631129–1131. [DOI] [PubMed] [Google Scholar]
- 35.Frick M, Ostergren J, Rosenqvist M. Effect of intravenous magnesium on heart rate and heart rate variability in patients with chronic atrial fibrillation. Am J Cardiol 199984104–108. [DOI] [PubMed] [Google Scholar]
- 36.Szyszka A, Wachowiak‐Baszynska H, Kaczmarek J.et al Magnesium protects myocardium during electric cardioversion but does not increase effectiveness. Kardiol Pol 19933898–101. [PubMed] [Google Scholar]
- 37.Ingemansson M P, Carlson J, Platonov P.et al Effects of MgSO4 and glucose, insulin and potassium (GIK) on atrial conduction during the first 12 hours after DC‐conversion of chronic atrial fibrillation. Scand Cardiovasc J 200135340–346. [DOI] [PubMed] [Google Scholar]
- 38.Ingemansson M P, Smideberg B, Olsson S B. Intravenous MgSO4 alone and in combination with glucose, insulin and potassium (GIK) prolong the atrial cycle length in chronic atrial fibrillation. Europace 20002106–114. [DOI] [PubMed] [Google Scholar]
- 39.Kobusiak‐Prokopowicz M, Mysiak A. The effect of intravenous magnesium on the arrhythmias in patients after electrical cardioversion. Pol Merkuriusz Lek 1999751–53. [PubMed] [Google Scholar]
- 40.Cybulski J, Kulakowski P, Budaj A.et al Intravenous amiodarone for cardioversion of recent‐onset atrial fibrillation. Clin Cardiol 200326329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cybulski J, Budaj A, Danielewicz H.et al A new‐onset atrial fibrillation: the incidence of potassium and magnesium deficiency. The efficacy of intravenous potassium/magnesium supplementation in cardioversion to sinus rhythm. Kardiol Pol 200460578–581. [PubMed] [Google Scholar]
- 42.Terrin N, Schmid C H, Lau J. In an empirical evaluation of the funnel plot, researchers could not visually identify publication bias. J Clin Epidemiol 200558894–901. [DOI] [PubMed] [Google Scholar]
- 43.Danias P G, Caulfield T A, Weigner M J.et al Likelihood of spontaneous conversion of atrial fibrillation to sinus rhythm. J Am Coll Cardiol 199831588–592. [DOI] [PubMed] [Google Scholar]
- 44.Cotter G, Blatt A, Kaluski E.et al Conversion of recent onset paroxysmal atrial fibrillation to normal sinus rhythm: the effect of no treatment and high‐dose amiodarone. A randomized, placebo‐controlled study. Eur Heart J 1999201833–1842. [DOI] [PubMed] [Google Scholar]
- 45.Ralston M A, Murnane M R, Kelley R E.et al Magnesium content of serum, circulating mononuclear cells, skeletal muscle, and myocardium in congestive heart failure. Circulation 198980573–580. [DOI] [PubMed] [Google Scholar]