Abstract
Objective
Approximately 2.8% of pregnancies are Ro/La antibody positive. 3–15% of fetuses develop complete heart block (CHB). First‐degree atrioventricular heart block (1° AVB) is reported in a third of Ro/La fetuses but as most have a normal postnatal ECG this may reflect inadequacies of Doppler measurement techniques.
Methods
Comparison was made between mechanical (mPR) and electrical (ePR) intervals obtained prospectively using Doppler and non‐invasive fetal ECG (fECG) in 52 consecutive Ro/La pregnancies in 46 women carrying 54 fetuses in an observational study at a fetal medicine unit.
121 mPR and 37 ePR intervals were recorded in 49 Ro/La fetuses. Five were referred with CHB and excluded. ePR was measured successfully in 35/37 (94%) and mPR was measured in all cases. 1° AVB was defined as PR >95% CI. Logistic regression predicted abnormal final fetal rhythm from first mPR or ePR.
Results
The ePR model gave 66.7% sensitivity (6 of 8 final abnormal fetal rhythm cases were predicted correctly in fetuses >20 weeks) and 96.2% specificity. mPR gave 44.4% sensitivity (4 of 9 cases) and 88.5% specificity. Z scores for ePR (zPR) were calculated from 199 normal fetuses. The area under the receiver operator characteristic (ROC) curve was 0.88 (95% CI, 0.754 to 1.007). A cut‐off of 1.65 gave a sensitivity of 87.5% and specificity of 95% for those with prolonged and normal ePR intervals, respectively.
Conclusion
zPR is better than mPR at differentiating between normal and prolonged PR intervals, suggesting that fECG is the diagnostic tool of choice to investigate the natural history and therapy of conduction abnormalities in Ro/La pregnancies.
Anti‐Ro or La antibody positive pregnancies (Ro/La) have been found in about 2.8% of the pregnant population. The most serious consequence of transplacental transfer of these antibodies to the fetus is complete heart block (CHB), which affects 3% of Ro/La pregnancies, with the risk rising to 15% in a subsequent pregnancy.1,2,3,4 In addition to the morbidity and mortality associated with pacing procedures in young infants,5,6 progressive myocardial fibrosis has been reported which affects long‐term cardiac function and may necessitate transplantation.7,8,9 The alloimmune process is thought to exert its effect in more than the 3% of fetuses affected by CHB with PR interval prolongation (first‐degree atrioventricular heart block or 1° AVB) described in up to a third of fetuses in studies using Doppler methods of measurement.10 As most babies have a normal outcome, with progression to CHB described in only a small proportion, there is some debate over whether these findings represent a transient biological response to the presence of antibodies or whether they may be due to inadequacies of the existing measurement techniques.11
A simple and robust method of monitoring the PR interval in affected pregnancies is required to enable studies to assess the extent and determinants of progression to CHB in Ro/La pregnancies. Such a tool would also permit the assessment of different treatment strategies designed to halt this progression and to minimise later myocardial damage.12
We report the use of a novel technique, non‐invasive fetal ECG (fECG), in a comparison of Doppler mechanical PR (mPR) and electrical PR (ePR) measurements and their ability to predict final rhythm in 52 consecutive Ro/La pregnancies studied prospectively.
Methods
The study was performed following ethical approval from the hospital's ethical committee and written consent was obtained. We recruited from women with positive anti‐Ro/La status managed in our institution who are referred for fetal echocardiography and offered serial Doppler monitoring from 16 to 32 weeks' gestation. All fetuses were studied using an Acuson Sequoia 512 machine interfaced to a curvilinear 6C2 transducer (Siemens Medical Solutions, Acuson Division, Mountainview, California, USA). Doppler traces showing mitral valve diastolic filling and left ventricular systolic ejection were stored digitally for subsequent analysis. Fetal heart rate was measured and the AV interval was measured from the onset of the mitral A wave (atrial systole) to the onset of aortic ejection (ventricular systole) within the same cardiac cycle as previously described.12,13 The AV interval was measured on three consecutive Doppler waveforms by two independent observers and the values averaged. Interobserver and intraobserver variability of this method has previously been assessed by the examiners.14
Either just before or just after the ultrasound examination the women had a non‐invasive fECG recording by a midwife from which ePR was measured. We have described the system and methodology fully elsewhere.15 The equipment used is portable and the size of a laptop computer. The women lay relaxed in a supine or lateral position and the skin was prepared to reduce the impedance by gentle excoriation of surface skin cells (3M Skinprep 2236). No prior knowledge of fetal position was required and 12 electrodes were placed over the maternal abdominal wall and connected to a 12‐bit multi‐channel digital recorder with a sampling rate of 512 Hz. Acquisition of fECG data took on average 15 minutes, including obtaining consent and skin preparation. About 2 minutes of data were recorded and from this, a 60‐second sample, free of artefact, was selected for processing. The data were processed using the QinetiQ signal‐processing algorithm and a parameterised fECG report was generated which included a 60‐second rhythm strip. Postnatal ECG was performed in all Ro/La babies and serial postnatal ECGs and a paediatric electrophysiology opinion was given in those showing fetal or postnatal abnormality.
Statistical analysis
Logistic regression was used to predict an abnormal (CHB or 1° or 2° AVB) final fetal rhythm from mPR or ePR. The resulting sensitivity, specificity and positive and negative predictive value of both methods were compared. Z scores for ePR were calculated from 199 fetuses used to create our previous normal ranges15 and a receiver operator characteristic (ROC) curve was used to assess the area under the curve and to obtain threshold values. Sensitivity and specificity of the resulting diagnostic rule based on the Z score for ePR (zPR) is reported.
Results
Demographics
Fifty‐two anti‐Ro/La pregnancies were examined in 46 women carrying 54 fetuses. The median maternal age was 31 (range 18–42) years and parity 0 (0–3). Five women are represented more than once in these data (one with three pregnancies and four with two pregnancies). All pregnancies were singleton apart from one triplet set. Thirty‐seven mothers had a history of active connective tissue disease: Sjögren's syndrome (SJS, 9); systemic lupus erythematosus (SLE, 25), and both SJS and SLE (3). However, no mother was on maintenance steroids during the pregnancy for connective tissue disease. Five women were referred in seven pregnancies because they had previously delivered a baby with CHB.
Five fetuses were referred with established CHB and were excluded from the study as the PR could not be measured. Two Ro/La pregnancies are included that were referred with CHB but showed at least temporary reversion to sinus rhythm (table 1: cases 5 and 7) permitting PR measurements to be made. The outcomes of these seven pregnancies and two further pregnancies affected by 1° AVB are shown in table 1.
Table 1 Outcome of fetuses showing atrioventricular heart block.
| Case | Referral gestation | Referral rhythm | Rhythm at delivery | Fetal therapy | Gestation delivery/death | Postnatal rhythm | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 27+5 | CHB | CHB | None | c/s 39 weeks | CHB | Not paced |
| 2 | 21+1 | CHB | CHB | None | c/s 38+1 | CHB | PPM 2.5 yrs |
| 3 | 24 | CHB | SSS | None | c/s premature labour 34 weeks | SSS | Hypothyroid on thyroxine. No pacing at 14 months |
| 4 | 22 | CHB | 1° AVB | Betamethasone 5 mg o.d. (1 month) | c/s 31 weeks IUGR (1.26 kg) | 2° AVB | PPM 1 year |
| 5 | 22 | CHB & hydrops | CHB IUD | None | IUD 31 weeks | IUD | IUD |
| 6 | 26+6 | CHB | CHB | None | c/s 39 2.7 kg | CHB | PPM day 4 |
| 7 | 20 | 2:1 heart block | CHB | Dexamethasone 6 weeks (SLE, IDDM, hypothyroid) | c/s 38 weeks (3.95 kg) | CHB | No Rx – moved abroad |
| 8 | 19 | 1° AVB | 1° AVB | None | SVD term | 1° AVB | None |
| 9 | 25+5 | 1° AVB | 1° AVB | None | SVD term | 1° AVB | None |
1° AVB, first‐degree heart block; 2° AVB, second‐degree heart block; CHB, complete heart block; c/s, caesarean section; IDDM, insulin‐dependent diabetes mellitus; IUD, intrauterine death; IUGR, intrauterine growth restriction; o.d., once daily; PPM, permanent pacemaker; Rx, treatment; SLE, systemic lupus erythematosus; SSS, sick sinus syndrome; SVD, spontaneous vaginal delivery.
There were three deaths: one an affected fetus that suffered an intrauterine death. The second was a fetus with normal serial PR intervals that had an unexpected intrauterine death at 34 weeks. A third was a growth‐restricted triplet with normal PR intervals that was stillborn.
Two fetuses had structural heart disease: both had normal situs and connections, one had an atrioventricular septal defect and the other had three small muscular ventricular septal defects. Neither was associated with heart block or chromosomal defects.
PR intervals
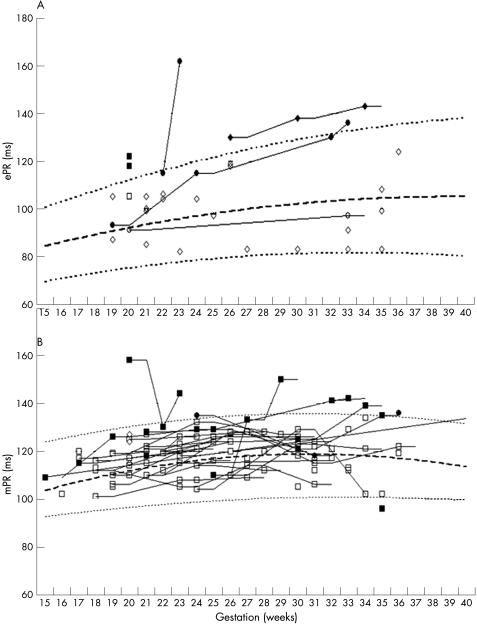
We recorded 121 mPR and 37 ePRs intervals in 49 Ro/La fetuses. The ePR readings were measured by observers blinded to the mPR results. Electrical separation of the PR interval was not possible in two of 37 fECG recordings (6%) but mPR was successful in all. A full fECG signature (including T waves) was obtained in 31 of the 35 (88.6%) separated signals. We have previously published reference ranges for fECG time intervals in a singleton cohort of 199 normal fetuses.15 A prolonged PR interval was defined as one lying above 95% gestational age‐related confidence intervals (95% CI). All mPR and ePR intervals measured in this study were plotted against published reference ranges (fig 1).13,15 In most cases the mPR was longer than ePR because it includes electromechanical delay and isovolumic contraction time.
Figure 1 (A) Electrical (ePR) and (B) mechanical Doppler (mPR) intervals for individual Ro/La fetuses plotted against published reference ranges for normal fetuses. Mean (dashed line) and 95% CI (dotted line) for the normal ranges. Shaded symbols represent individuals with mPR or ePR values that are, or that rise above the 95th centile during pregnancy.
The interobserver and intraobserver variability for both methods has been published previously by our examiners and demonstrated 95% limits of agreement (interobserver and intraobserver) of −9.9 to +15.3 ms and −11.3 to + 9.4 ms for the Doppler method and −3.9 to +3.1 ms and −2.5 to +1.1 ms for fECG measurements.14
A prolonged mPR and/or ePR were recorded in seven fetuses, representing 14% of the cohort (7/49) and their outcome is shown in table 2. Four babies (4/7) had normal postnatal ECGs. Two of the three with abnormal recordings had 1° AVB confirmed postnatally and the third suffered intrauterine death from CHB and hydrops. Post‐mortem examination of the conduction tissue confirmed diffuse fibrosis.
Table 2 Serial measurements and outcome of seven fetuses with paired measurements showing abnormal ePR and/or mPR.
| Pregnancy | Gestation (weeks) | Heart rate (bpm) | mPR (ms) | ePR (ms) | Final fetal rhythm | Outcome |
|---|---|---|---|---|---|---|
| 1 | 22.0 | 141 | 130 | 115 | CHB | Hydrops & IUD |
| 23.0 | 153* | 144 | 162 | |||
| 2 | 24.0 | 146 | 129 | 115 | 1° AVB | 1° AVB |
| 32.1 | 153 | 141 | 130 | |||
| 33.0 | 138 | 142 | 136 | |||
| 3 (Triplet 1) | 19.7 | 156 | 127 | 118 | SR | Normal ECG |
| 3 (Triplet 2) | 19.7 | 153 | 124 | 122 | SR | Normal ECG |
| 4 | 25.7 | 139 | 127 | 130 | 1° AVB | 1° AVB |
| 29.7 | 136 | 125 | 138 | |||
| 33.7 | 132 | 139 | 143 | |||
| 5 | 21.3 | 149 | 128 | 100 | SR | Normal ECG |
| 35.4 | 140 | 135 | 108 | |||
| 6 | 36.0 | 140 | 134 | 124 | SR | Normal ECG |
Values in bold are >95th centile.
*Second‐degree heart block with heart rate ranging from 45 to 159 during the observation period.
1° AVB, first degree heart block; CHB, complete heart block; IUD, intrauterine death; SR, sinus rhythm.
Paired mPR and ePR recordings
There were 35 paired recordings in 24 fetuses (70 recordings). Nineteen of these 70 recordings showed a prolonged PR in one or both measures (27%). Figure 1 shows values for ePR and mPR in individual fetuses. Of the 19 abnormal test results, six (in four individuals) were normal postnatally and 13/70 recordings in three individuals were truly prolonged. This gave sensitivity, specificity, positive and negative predictive values for a single test result of: 100%; 93%; 80% and 100% for ePR and 63%; 85%; 56%; 88% for mPR, respectively.
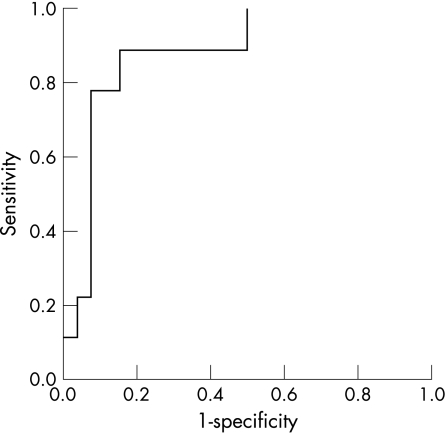
To assess the ability of the first measured interval to predict final fetal rhythm, a logistic regression model was created. This was based on the ePR of paired tests (8 abnormal traces from 3 fetuses and 26 normal traces from 15 fetuses) and resulted in a model with 66.7% sensitivity (6 out of 8 final abnormal fetal rhythms predicted correctly) and 96.2% specificity. A similar model based on mPR had 44.4% sensitivity (4 out of 9) and 88.5% specificity. We compared these results with Z scores for ePR calculated from previously published results for normal fetuses >20 weeks' gestation15 and created a ROC curve to assess threshold values, and sensitivity and specificity of the resulting diagnostic rule (fig 2). The curve was calculated from 28 observations (8 abnormal traces from 3 fetuses and 20 normal traces from 15 fetuses). The area under the curve was 0.88 (95% CI 0.754 to 1.007). A cut‐off of 1.65 gave a sensitivity of 87.5% and specificity of 95% for those with prolonged and normal ePR intervals, respectively.
Figure 2 Receiver operator characteristic curve for Z scores for electrical PR (zPR).
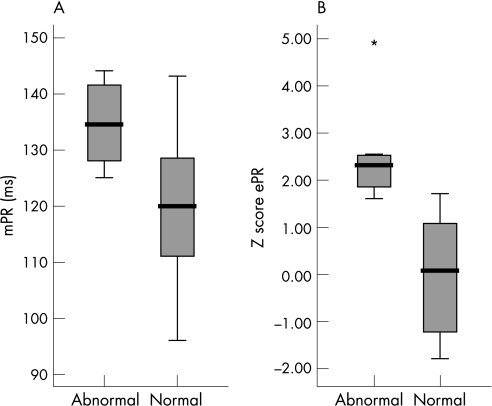
The box and whisker plots (fig 3) demonstrate that zPR is better than mPR in differentiating between normal and prolonged PR. Where both methods showed prolonged PR (6 pairs in 4 fetuses), 3 fetuses had 1° AVB or CHB postnatally and in fetuses showing prolonged ePR alone (4 recordings in 3 fetuses), 2 of 3 fetuses had persistent 1° AVB in infancy. In contrast, the two babies with prolonged mPR alone (3 recordings in 2 fetuses) had normal postnatal ECGs.
Figure 3 Box and whisker plots demonstrate that Z scores for (B) electrical PR (zPR) are better than (A) Doppler mechanical PR (mPR) in differentiating between normal and prolonged PR. *p<0.05.
Fetal therapy
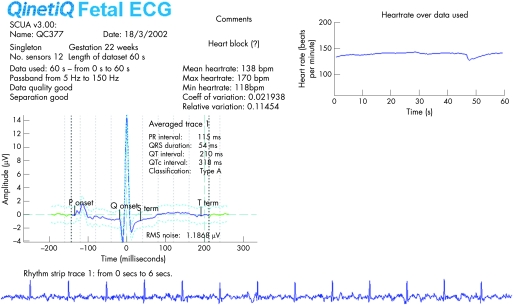
This study was not designed to assess the role of steroids or their effectiveness in the prevention of progression of lesser degrees of heart block to CHB. High‐dose steroids were used in three mothers: two whose fetuses already had evidence of block and a third treated prophylactically because she had had two previously affected fetuses. Noteworthy observations were made in two fetuses. The first (table 1, case 5) reverted to apparent sinus rhythm with resolution of hydrops spontaneously, following which steroid therapy was started to prevent deterioration. The fECG documents the spontaneous rise in heart rate to 138 bpm at 22 weeks' gestation but both ePR and mPR were >95% CI, confirming 1° AVB (fig 4). The 60‐second rhythm strip confirms this was not a junctional tachycardia in the setting of CHB. Within 2 weeks 2° AVB (fig 5) then CHB occurred despite steroid therapy (fig 6) and the fetus suffered an intrauterine death at 31 weeks. A second mother (table 1, case 4) was treated with 5 mg betamethasone daily for 4 weeks after diagnosis of fetal CHB (heart rate 78 bpm and a small pericardial effusion at 25 weeks). No fECG was available but she appeared to be in sinus rhythm (heart rate: 144 to 158) at repeat fetal echo 1 week later and remained so throughout pregnancy but the mPR increased from 110 to 133 ms and then to 150 ms by 29 weeks confirming 1° AVB. Because of growth restriction, steroids were discontinued and the heart rate remained stable throughout pregnancy. However, 2° AVB was confirmed following episodes of intermittent bradycardia on the neonatal intensive care unit. This progressed during infancy and the baby was paced by a year.
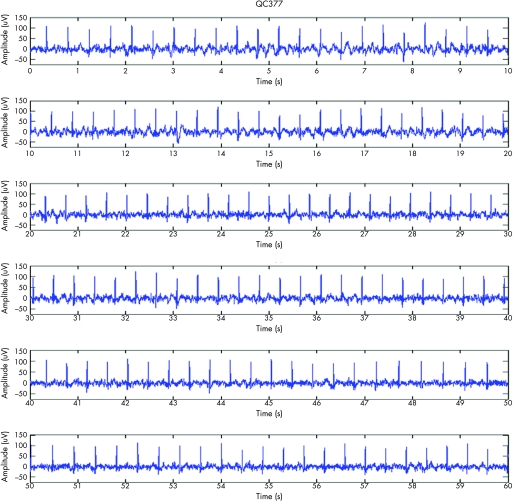
Figure 4 Complete fetal ECG (fECG).
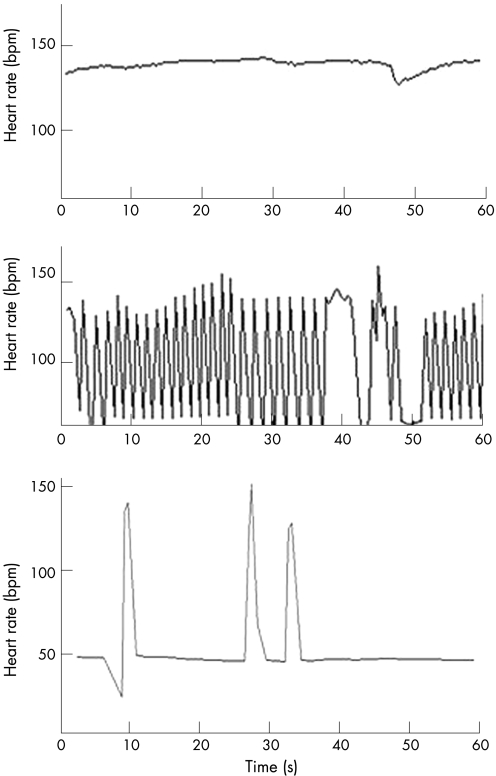
Figure 5 60‐second rhythm strip documenting the spontaneous conversion from complete heart block (CHB) to sinus rhythm. The ePR was >95% CI confirming first‐degree heart block.
Figure 6 Beat to beat R–R intervals over 60 seconds taken from the serial fECGs recorded at 22, 23 and 24 weeks' gestation show progression to 2° atrioventricular heart block, then complete heart block despite steroid therapy commenced at 22 weeks' gestation.
Discussion
We have shown in this study that zPR is better than mPR at differentiating between normal and prolonged PR in the fetus. This is in agreement with our previous report that the reproducibility of the PR interval using non‐invasive fECG shows tighter limits of agreement than Doppler methods.14
We believe our study design permits us to answer some questions about the natural history of PR prolongation in Ro/La pregnancies, and its relationship with CHB.
The reconciliation between the high prevalence of fetal 1° AVB with normal postnatal PR intervals has been proposed due to a transient biological response to the transplacental transfer of Ro/La antibodies.10,11 However, the comparatively poor specificity and positive predictive value of mPR in our study suggests that the variability inherent in the method used to measure mPR intervals is likely to be important.12,13 The sensitivity of the fECG technique was superior to Doppler methods as no individual with an abnormal outcome had normal fECG recordings after 19 weeks, whereas two fetuses with 1° AVB recognised on fECG at their first visit (at 24 and 25.7 weeks' gestation) had normal mPR intervals recorded on serial studies until 32.1 and 33.7 weeks, respectively. The uncertainty of robustness of mPR interval is reflected in the literature which suggests using 150 ms as a useful cut‐off to predict abnormality.11,16 From our experience we would recommend using age‐related centiles but recognise that mild elevation of time intervals above the 95th centile in both methods (but <140 ms) account for the majority of false positive values observed in our study. We therefore conclude that the use of age‐related intervals is more appropriate than an absolute cut‐off value; however, if a cut‐off value is required then 140 ms may be more appropriate than 150 ms.
The fECG method used in this study allows beat‐to‐beat recording of the fetal heart and so, unlike previous methods of recording the fetal ECG, we can record rhythm strips of any length in the human fetus. In addition, each electrode sited on the maternal abdomen detects a mathematical combination of signals from the fetal heart and produces an energy map permitting localisation of each individual fetus. In multiple pregnancies, such as in the triplet case in this study, this permits accurate pairing of the individual ePR and mPR traces (fig 7).
Figure 7 Energy maps produced from the location of individual fetal heart sources for the triplet pregnancy. The strength of an individual fECG signature is shown at each of the maternal abdominal electrodes and strength of the overall signal is demonstrated graphically by the coloured areas.
Several Doppler methods of AV interval assessment have been described: paired tracings of the mitral valve (MV) and aorta (Ao) and the superior caval vein (SCV) and Ao Doppler and across pulmonary and tricuspid valves which may give an AV interval closer to the fECG PR measurement as it does not incorporate the inter‐atrial conduction time. In a comparative study of MV/Ao and SCV/Ao methods no systematic differences were revealed.13 One advantage of the SCV/Ao method is that it is successful at fast heart rates unlike the MV/Ao Doppler tracings where technical success is less at faster fetal heart rates (usually >160 bpm) because of summation of E and A waves. We used Doppler of MV/Ao (in preference to SCV/Ao) to evaluate the mPR interval because we found it easier to obtain. Furthermore, this measure is an expression of left‐sided cardiac events.
In contrast to other studies, the three mothers in this study who received high‐dose steroid therapy for fetal heart block showed maternal and fetal complications including diabetes, oligohydramnios and growth restriction, without clear evidence of benefit to fetal cardiac conduction or function, although hydrops resolved in one.17 We apply caution in prescribing steroid therapy and do not treat unless there is evidence of second‐degree heart block or hydrops. However, our experience does not support a role for steroids alone in the antenatal management of congenital fetal heart block due to anti‐Ro/La antibodies.
Limitations
The fECG equipment was not available in the early years of the study (before 2001) and for a period during the middle of the study, thus the number of paired traces is limited to 37 recordings (in 26 fetuses) and lack of separation in two recordings reduced the sample size to 35 (in 24 fetuses). The number of cases included in the statistical model was confined to those over 20 weeks' gestation as traces from earlier gestational ages resulted in more predictive errors, possibly due to the paucity of data on fetal ePR intervals from which to create robust Z scores. Simultaneous mPR and ePR recordings were not possible but there was a delay of less than 5 minutes between measurements in those with paired traces and no significant difference in fetal heart rate using the two methods. We have therefore assumed that the fetus remained in a stable condition for both recordings. The statistical model underestimates the sensitivity of both methods as it tests the ability for the first fetal PR interval to predict the final rhythm. In reality, transplacental transfer of antibodies may occur between 16 and 32 weeks, but most commonly in the second trimester and thus early measurements may be normal, as documented in this study. The known variability of the clinical course in this disease reduces the utility of a ROC curve in gestational ages below 20 weeks.
Conclusions
Both techniques showed good agreement in identifying Ro/La fetuses with increased PR intervals but the reproducibility, sensitivity and specificity of fECG in this small series was superior. This supports our conclusion that fECG would be the diagnostic tool of choice to use in any trial to investigate both the natural history and therapy of conduction abnormalities in Ro/La pregnancies.18 Additional benefits are that it is independent of operator use and fetal number or lie. Moreover, the gold standard for the diagnosis of conduction defects in the neonate is the ECG (either surface or transoesophageal) and it seems more logical to use fetal electrical cardiac signals than any existing mechanical surrogate.
Acknowledgements
We would like to acknowledge QinetiQ Ltd, Centre for Signal and Information Processing, QinetiQ, Malvern, Worcestershire for loan of the fECG equipment
Abbreviations
1° AVB - first‐degree atrioventricular heart block
CHB - complete heart block
fECG - fetal ECG
ROC - receiver operator characteristic
Footnotes
Funding: Research midwife Salome Oseku‐Afful recruited pregnant women and performed the fECG and William Dennes aided in data collection and processing and both were supported by echoUK (the Tiny Tickers charity) (http://www.echocharity.org.uk).
Competing interests: MT is employed by QinetiQ.
References
- 1.Taylor P V, Scott J S, Gerlis L M.et al Maternal antibodies against fetal cardiac antigens in congenital complete heart block. N Engl J Med 1986315667–672. [DOI] [PubMed] [Google Scholar]
- 2.Ho S Y, Esscher E, Anderson R H.et al Anatomy of congenital complete heart block and relation to maternal anti‐Ro antibodies. Am J Cardiol 198658291–294. [DOI] [PubMed] [Google Scholar]
- 3.Buyon J P, Winchester R J, Slade S G.et al Identification of mothers at risk for congenital heart block and other neonatal lupus syndromes in their children. Comparison of enzyme‐linked immunosorbent assay and immunoblot for measurement of anti‐SS‐A/Ro and anti‐SS‐B/La antibodies. Arthritis Rheum 1993361263–1273. [DOI] [PubMed] [Google Scholar]
- 4.Machado M V, Tynan M J, Curry P V.et al Fetal complete heart block. Br Heart J 198860512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groves A M, Allan L D, Rosenthal E. Outcome of isolated congenital complete heart block diagnosed in utero. Heart 199675190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balmer C, Fasnacht M, Rahn M.et al Long‐term follow up of children with congenital complete atrioventricular block and the impact of pacemaker therapy. Europace 20024345–349. [DOI] [PubMed] [Google Scholar]
- 7.Clancy R M, Buyon J P. Autoimmune‐associated congenital heart block: dissecting the cascade from immunologic insult to relentless fibrosis. Anat Rec A Discov Mol Cell Evol Biol 20042801027–1035. [DOI] [PubMed] [Google Scholar]
- 8.Buyon J P, Clancy R M. From antibody insult to fibrosis in neonatal lupus—the heart of the matter. Arthritis Res Ther 20035266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nield L E, Silverman E D, Taylor G P.et al Maternal anti‐Ro and anti‐La antibody‐associated endocardial fibroelastosis. Circulation 2002105843–848. [DOI] [PubMed] [Google Scholar]
- 10.Sonesson S E, Salomonsson S, Jacobsson L A.et al Signs of first‐degree heart block occur in one‐third of fetuses of pregnant women with anti‐SSA/Ro 52‐kd antibodies. Arthritis Rheum 2004501253–1261. [DOI] [PubMed] [Google Scholar]
- 11.Buyon J P, Askanase A D, Kim M Y.et al Identifying an early marker for congenital heart block: when is a long PR interval too long? Arthritis Rheum 2005521341–1342. [DOI] [PubMed] [Google Scholar]
- 12.Dancea A, Fouron J C, Miro J.et al Correlation between electrocardiographic and ultrasonographic time‐interval measurements in fetal lamb heart. Pediatr Res 200047324–328. [DOI] [PubMed] [Google Scholar]
- 13.Andelfinger G, Fouron J C, Sonesson S E.et al Reference values for time intervals between atrial and ventricular contractions of the fetal heart measured by two Doppler techniques. Am J Cardiol 2001881433–6, A8. [DOI] [PubMed] [Google Scholar]
- 14.Pasquini L, Seale A, Belmar C.et al PR interval: a comparison of electrical and mechanical methods in the fetus. Early Hum Dev . 2007;83231–237. [DOI] [PubMed]
- 15.Taylor M J, Smith M J, Thomas M.et al Non‐invasive fetal electrocardiography in singleton and multiple pregnancies. BJOG 2003110668–678. [PubMed] [Google Scholar]
- 16.Glickstein J S, Buyon J, Friedman D. Pulsed Doppler echocardiographic assessment of the fetal PR interval. Am J Cardiol 200086236–239. [DOI] [PubMed] [Google Scholar]
- 17.Jaeggi E T, Fouron J C, Silverman E D.et al Transplacental fetal treatment improves the outcome of prenatally diagnosed complete atrioventricular block without structural heart disease. Circulation 20041101542–1548. [DOI] [PubMed] [Google Scholar]
- 18.Jaeggi E T, Hamilton R M, Silverman E D.et al Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. A single institution's experience of 30 years. J Am Coll Cardiol 200239130–137. [DOI] [PubMed] [Google Scholar]