Abstract
Aims
To evaluate the effect of a disease management programme for patients with coronary heart disease (CHD) and chronic heart failure (CHF) in primary care.
Methods
A cluster randomised controlled trial of 1316 patients with CHD and CHF from 20 primary care practices in the UK was carried out. Care in the intervention practices was delivered by specialist nurses trained in the management of patients with CHD and CHF. Usual care was delivered by the primary healthcare team in the control practices.
Results
At follow up, significantly more patients with a history of myocardial infarction in the intervention group were prescribed a beta‐blocker compared to the control group (adjusted OR 1.43, 95% CI 1.19 to 1.99). Significantly more patients with CHD in the intervention group had adequate management of their blood pressure (<140/85 mm Hg) (OR 1.61, 95% CI 1.22 to 2.13) and their cholesterol (<5 mmol/l) (OR 1.58, 95% CI 1.05 to 2.37) compared to those in the control group. Significantly more patients with an unconfirmed diagnosis of CHF had a diagnosis of left ventricular systolic dysfunction confirmed (OR 4.69, 95% CI 1.88 to 11.66) or excluded (OR 3.80, 95% CI 1.50 to 9.64) in the intervention group compared to the control group. There were significant improvements in some quality‐of‐life measures in patients with CHD in the intervention group.
Conclusions
Disease management programmes can lead to improvements in the care of patients with CHD and presumed CHF in primary care.
Cardiovascular diseases including coronary heart disease (CHD) and chronic heart failure (CHF) are the main cause of morbidity and mortality in most European countries.1 Mortality from cardiovascular disease has declined over the last 30 years, a trend which has been attributed to secondary prevention therapies.2,3 However, European surveys have shown considerable potential for improved levels of secondary prevention in people with established CHD.4 Studies in primary care, where most of these patients are managed, have also reported considerable potential to further increase secondary prevention through medical and lifestyle interventions.5,6 “Medical” measures include aspirin therapy and blood pressure and lipid control, while “lifestyle” measures include increased exercise, dietary modification and smoking cessation.5 CHF is also a highly prevalent, chronic condition with high mortality and morbidity. It is increasing in prevalence and the public health burden from CHF is therefore likely to rise substantially over the next 10 years.7 The quality of life of patients with CHF is worse than for most chronic conditions managed in primary care and five‐year survival is worse than for many malignant conditions.8 However, appropriate treatment, including inhibitors of the renin‐angiotensin‐aldosterone system and beta‐blockers, has the potential to reduce hospitalisation and mortality in these patients.9,10 The task of implementing a comprehensive package of effective measures for large numbers of patients has been described as daunting.5 It is therefore important to develop implementation strategies that are practical and effective. Many patients with CHF are incorrectly diagnosed and inadequately treated in primary care11 and obstacles to appropriate primary care management include lack of knowledge, fear of complications with pharmacological treatments, lack of time and limited facilities for investigations.12,13
Systematic reviews indicate that secondary prevention programmes improve the process of care, reduce admissions to hospital and enhance quality of life or functional status in patients with CHD.14 Similarly, systematic reviews of disease management programmes in CHF suggest that specialised, multidisciplinary follow‐up can reduce hospitalisation and may lead to cost saving.15,16,17 However, all the CHF trials included in these systematic reviews were conducted in highly specialised centres and recruited patients following discharge after hospitalisation. The applicability of the available CHF management programmes to countries with a primary care‐based healthcare system has therefore recently been questioned.18
To achieve improved secondary prevention of CHD and CHF, primary care will need to adopt a systematic approach. Although disease management clinics for the management of CHD in primary care can improve patients' outcomes,5 there are no such studies in the management of patients with CHF. Since the majority of patients with CHF will also have CHD,19 we investigated the effect of a disease management programme for patients with either or both conditions in primary care.
Methods
Practice recruitment and randomisation
This was a cluster randomised controlled trial with randomisation at practice level. All primary care practices in one region in the city of Leicester, UK were invited to participate. A randomisation procedure based on case‐control pairs was selected to promote similarity between the general practices included in the two study groups. Twenty volunteer practices (with 53 general practitioners) were randomly allocated to one of two study arms using computer‐generated case‐control pairs. Each pair of practices had been matched as closely as possible in terms of list size, number of general practitioner partners, Jarman score (as an indicator of deprivation) and teaching and training status of the practice. The study was approved by the local research ethics committee and all participants gave written, informed consent to participation.
Interventions and patient recruitment
Patients were identified from each practice database using disease registers and medication searches. Patients were included if a diagnosis of CHD (angina or past medical history of myocardial infarction) or CHF was specifically recorded or was suggested by prescribed medication. In the intervention group, two peripatetic nurse specialists trained in the management of CHD and CHF travelled between practices, where they held weekly clinics. The nurse intervention included patient assessment, confirmation of diagnosis by investigations, medication management and titration, home visits for housebound patients with CHF and liaison between primary and secondary care. The nurses had the facility to refer patients for echocardiography and for assessment in a secondary care cardiology clinic. This clinic offered echocardiography, assessment by a senior cardiologist and, where considered appropriate, access to additional investigations including exercise electrocardiography, Holter electrocardiography, stress echocardiography and coronary angiography. In the intervention practices, patients with a presumed diagnosis of CHF which had not been objectively confirmed were assessed clinically and had an ECG. These patients were considered for referral for an echocardiogram on the basis of medical history, signs, symptoms and an abnormal ECG.20 Patients in the control group received usual care from members of the primary healthcare team. However, control group practices were provided with the same open access echocardiography and access to the secondary care cardiology clinic serving the intervention group practices. Patients from both groups were approached by letter from their general practitioner inviting them to participate, together with a consent form and a baseline questionnaire. Intervention group patients were offered an appointment to see the specialist nurse at their general practice. For patients in both groups, consent to participate in the study included permission for review of their general practice records. All patients were followed up for 12 months from the date of recruitment.
Outcome measures
The study had three primary outcome measures: the proportion of patients with a history of myocardial infarction receiving a beta‐blocker;21 in patients with CHD, a recorded serum cholesterol less than 5 mmol/l in the previous year,21,22 and the proportion of patients with left ventricular systolic dysfunction being treated with an ACE inhibitor.23,24 Secondary outcomes in patients with CHD included process of care measures, body mass index and level of blood pressure control. Secondary outcome measures relating to CHF included the proportion of patients with a presumed diagnosis of CHF having an echocardiogram and the proportion of patients having confirmation or rejection of the diagnosis of left ventricular systolic dysfunction by an echocardiogram.
We also measured quality of life using a generic questionnaire (SF‐36)25 in all patients and also disease‐specific questionnaires. We used the Seattle Angina Questionnaire26 in people with angina. This instrument has been demonstrated to be valid, reproducible and sensitive to clinical change.26 We used the Left Ventricular Dysfunction questionnaire (LVD‐36) in patients with CHF.27 This provides a short, simple, valid and reliable measure of health status in patients with left ventricular dysfunction. The instrument has been shown to have a high level of reliability and validity, and appears to measure changes in health. Better quality of life is indicated by higher SF‐36 and Seattle Angina Questionnaire scores and lower LVD‐36 scores.
Data collection
Baseline and follow‐up data were collected from the general practice records of all patients recruited, including any who failed to return follow‐up questionnaires. A structured data collection form had been prepared, including piloting in one practice. Data collected included information about process of care indicators such as recording of smoking status; outcome measures such as blood pressure levels; and detailed information including prescribing data for estimation of costs. Four nurse data collectors were recruited and trained to carry out data extraction. To promote reliability in the data collection, regular review meetings were held with the data collectors throughout the collection period, at which queries and problems encountered were discussed. Towards the end of the data collection exercise, a reliability check was carried out for which each of the four data collectors independently extracted data from the same three sets of patient notes at one general practice.
Sample size
Sample size calculations were carried out for the two subsets of patients with CHD and with CHF, in order to estimate numbers needed in relation to our three primary outcome measures. Using a cluster sampling procedure with a cluster size of 20 and allowing for a background rate of 35% of post‐myocardial patients being treated with a beta‐blocker in the control group and an intra‐cluster correlation coefficient of 0.06,28 the number of patients required to detect an increase in the proportion treated to 55% with 80% power (Type I Error: 0.05) is 190 from a total of 20 practices. We estimated that 35% of patients with CHD would have a cholesterol level <5 mmol/l.29 Sensitivity analysis was carried out assuming an intra‐cluster correlation coefficient between 0.05 and 0.06 with a total of 20 practices. We estimated that a sample of 654 to 715 patients would be required to show an increase of 30% in the proportion of CHD patients with a cholesterol level <5 mmol/l. For patients with CHF, we estimated that 30% of patients with left ventricular systolic dysfunction would be treated with an ACE inhibitor.20 Assuming an intra‐cluster correlation coefficient of 0.05 to 0.06 and a follow‐up rate of 60% of patients on an ACE inhibitor, we estimated that we would require between 118 and 121 patients. Assuming that only 50% of patients in primary care having CHF would have had an echocardiogram, we required approximately 240 patients with a presumed diagnosis of CHF in order to obtain 120 confirmed cases.
Analysis
We carried out statistical analyses according to CONSORT guidelines for cluster randomised trials. The categorical variables were presented as number (percentage) and the continuous study variables were presented by appropriate measure of central tendency and dispersion. The baseline and follow‐up characteristics were compared using appropriate non‐parametric tests. To test the trend of the patients' dichotomous responses between the intervention and control groups, we used the random effects model with logit link with adjustments for the cluster effects and baseline information. Mixed‐effect regression models under Gaussian distribution and identity link function were used to analyse the effect of the intervention on continuous clinical outcomes. Robust standard errors of the regression coefficients were estimated and the adjusted means were based on linear prediction. Apart from adjusting for cluster effects and baseline information, we also explored the possible effects of confounding factors including age, gender and deprivation scores. Owing to the problem of non‐normality of quality‐of‐life measures, we adopted the non‐parametric bias corrected bootstrap technique for hypothesis testing and estimation of relevant parameters. All analysis was carried out on an intention‐to‐treat basis with missing values replaced with the last recorded values and p values are presented with four places of decimal in the tables. We used SAS 9.2 and STATA 8 for the analysis.
Follow‐up Seattle Angina Questionnaire scores on all the five scales were adjusted for baseline scores and cluster effects. The effects of age and gender were also explored while comparing between the intervention and control groups. The percentage scores from the Left Ventricular Dysfunction (LVD) Questionnaire were analysed using a random effects model with adjustments for baseline scores, potential risk factors and clustering. SF Health Outcomes Scoring Software (QualityMetric Incorporated) was used for the scoring of SF‐36 outcomes, including missing data estimation.
Results
Practice characteristics and study progress
Intervention and control group practices were well matched overall. The number of primary care physicians per practice was similar (four single‐handed practices in the intervention group and five in the control group), as were the average number of patients per practice (intervention group 4823, control group 4657) and mean practice Jarman score (intervention group +19.8, control group +20.9). There were two teaching practices in the intervention group and one in the control group and each group had one practice with a CHD nurse.
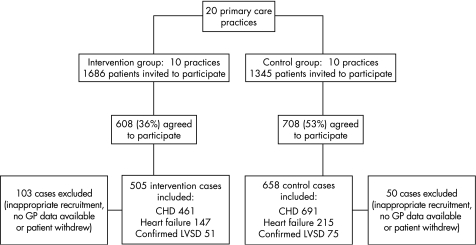
Recruitment to the trial between 2001 and 2002 resulted in a sample of 1316 patients (fig 1). This total comprised 608 intervention group patients and 708 controls. A total of 103 patients in the intervention group were excluded from the analysis: 98 because of inappropriate recruitment (acute referrals to the cardiology nurse by the general practitioner or no history of either CHD or CHF found on review of patient records); 4 because no general practice records were available for review and 1 patient withdrew from the study. In the control group, 50 cases were excluded: 33 ineligible patients, 15 for whom records were unavailable and 2 who withdrew. The final sample of 1163 cases for the intention‐to‐treat analysis therefore included 505 intervention group patients and 658 controls. This included 54 patients (intervention group 39, control group 15) who did not complete the trial per protocol as they died or left the practice during the follow‐up period. For these cases, the latest results available were used in place of 12‐month follow‐up data. Table 1 shows the characteristics of intervention and control group participants in the study and indicates that overall the two groups were reasonably well matched in spite of some differences including a higher proportion of male patients in the intervention group.
Figure 1 Flow of participants through the trial. CHD, coronary heart disease; LVSD, left ventricular systolic dysfunction.
Table 1 Baseline characteristics of patients included in ITT analysis.
| All patients | Patients with CHD | Patients with presumed diagnosis of CHF | ||||
|---|---|---|---|---|---|---|
| Intervention group (n = 505) | Control group (n = 658) | Intervention (n = 461) | Control (n = 619) | Intervention (n = 147) | Control (n = 215) | |
| Median (Q1, Q3) age in years | 70 (64, 77) | 71 (63, 78) | 70 (63, 76) | 71 (63, 78) | 72 (65, 79) | 75 (67, 81) |
| Male gender | 341 (68)* | 384 (58)* | 316 (69)* | 370 (60)* | 97 (66)* | 119 (55)* |
| Mean multiple deprivation score | 39.04 (16.66) | 39.81 (15.01) | 38.74 (16.63) | 39.67 (15.04) | 39.16 (15.66) | 41.25 (14.01) |
| Angina | 435 (86) | 588 (89) | 435 (94) | 588 (95) | 100 (68)* | 167 (78)* |
| Mean years since diagnosis | 7.00 (6.39) | 6.68(5.97) | 7.00 (6.39) | 6.68 (5.97) | 7.08 (6.97) | 7.71 (6.69) |
| Past myocardial infarction | 213 (42) | 276 (42) | 213 (46) | 276 (45) | 67 (46) | 116 (54) |
| Mean years since diagnosis | 9.32 (7.55) | 8.42 (7.00) | 9.32 (7.55) | 8.42 (7.00) | 8.05 (8.02) | 8.59 (7.00) |
| Presumed heart failure | 147 (29) | 215 (33) | 103 (22)* | 177 (29)* | 147 (100) | 215 (100) |
| Mean years since diagnosis | 3.37 (3.80)* | 4.68 (3.54)* | 3.44 (3.96)* | 4.61 (3.41)* | 3.37 (3.80)* | 4.67 (3.53)* |
| Diabetes | 84 (17) | 151 (23) | 76 (16) | 140 (23) | 30 (20) | 60 (28) |
| Peripheral vascular disease | 38 (8) | 46 (7) | 35 (8) | 40 (6) | 18 (12) | 25 (12) |
| Hypertension | 258 (51) | 361 (55) | 237 (51) | 333 (54) | 84 (57) | 144 (67) |
| Mean systolic BP | 137.88 (18.37) | 138.80 (19.86) | 138.09 (18.23) | 138.51 (19.69) | 134.97 (18.62) | 137.26 (18.27) |
| Mean diastolic BP | 78.71 (9.92) | 78.33(9.88) | 78.86 (9.65) | 78.30 (9.93) | 76.80 (11.84) | 77.66 (9.23) |
| Total cholesterol level | 4.91 (0.98) | 5.01 (1.05) | 4.90 (0.99) | 5.00 (1.05) | 4.77 (0.86) | 5.00 (0.98) |
| Recorded as smokers | 55 (11)* | 110 (17)* | 51 (11)* | 104 (17)* | 17 (12)* | 32 (15)* |
| SF‐36 dimension scores | ||||||
| Physical functioning | 49.86 (28.99) | 46.91 (30.10) | 51.04 (29.09) | 47.69 (30.04) | 38.18 (27.38) | 35.53 (27.91) |
| Role physical | 37.30 (42.49) | 40.00 (44.71) | 39.01 (42.89) | 40.98 (44.90) | 24.45 (36.70) | 26.84 (40.39) |
| Bodily pain | 59.34 (28.57) | 55.91 (29.19) | 59.66 (28.44) | 55.78 (29.25) | 53.62 (27.29) | 50.41 (28.67) |
| General health mean score | 48.36 (23.60)* | 45.02 (24.04)* | 49.14 (23.76)* | 45.34 (24.09)* | 42.80 23.12) | 39.81 (23.02) |
| Vitality mean score | 46.02 (21.91) | 43.82 (23.42) | 46.91 (21.99) | 44.18 (23.50) | 39.52 (20.92) | 37.36 (20.54) |
| Social functioning mean score | 68.01 (29.60) | 65.62 (31.12) | 68.42 (29.91) | 66.11 (30.89) | 60.19 (30.16) | 58.47 (30.87) |
| Role emotional mean score | 53.71 (44.91) | 53.10 (45.40) | 54.70 (44.51) | 54.13 (45.47) | 42.35 (45.25) | 45.77 (45.54) |
| Mental health mean score | 70.75 (20.18)* | 67.31 (20.86)* | 70.82 (20.48)* | 67.65 (20.77)* | 70.85 (17.68) | 66.39 (18.44) |
Number (%) or mean (SD) except where otherwise indicated.
*p<0.05 Bootstrapped p values for quality‐of‐life measures.
Q1, first quartile; Q3, third quartile.
Secondary prevention
Table 2 shows the proportions of patients with CHD assessed for coronary risk factors (including blood pressure measurement, smoking status and body mass index or weight assessment) at baseline and after 12 months follow‐up. The table shows relatively low levels of assessment for each of these factors apart from blood pressure. In spite of an overall proportion of 82% (823/1012) with an assessment of blood pressure during the year before recruitment, the proportion increased to a greater extent in the intervention group (p⩽0.01). Moreover, at follow‐up, significantly more patients in the intervention group had adequate blood pressure control (<140/85 mm Hg; (p⩽0.01). Other CHD risk factors also showed greater improvement in the intervention group. Of the 51 patients with CHD recorded as smokers at baseline, 34 (67%) had a smoking cessation intervention (advice, referral to a smoking cessation service or nicotine replacement therapy prescribed) recorded during the follow‐up year, compared to 47/104 (45%) smokers in the control group (p<0.01). Serum cholesterol of <5 mmol/l was more commonly achieved in the intervention group (p = 0.03). Upwardly rounded unadjusted numbers needed to treat (NNT) for patients with adequate control of blood pressure and cholesterol are 8 (95% CI 6 to 16) and 10 (6 to 25), respectively. Table 3 shows the marginal mean values of systolic and diastolic blood pressure, total cholesterol and body mass index in the two groups. After adjustment for statistically significant confounders including age, gender, deprivation score and current smoking status, and including adjustment for baseline performance and cluster effects, blood pressure and cholesterol were lower in the intervention group (p⩽0.01, p = 0.01, respectively).
Table 2 Number of patients with CHD with appropriate assessment and treatment with specified drugs for secondary prevention at baseline and after 1 year of follow‐up.
| Number of observations | Number (%) | Estimated intra‐cluster correlation coefficients* (SE) | OR (95% CI)* | p Value | |||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| Risk factors | |||||||
| BP measured | |||||||
| Baseline | 1012 | 363 (81.8) | 460 (81.0) | ||||
| Follow‐up | 1058 | 446 (99.1) | 514 (84.5) | 0.1259 (0.0476) | 22.61 (6.47 to 70.13) | <0.0001 | |
| Cholesterol measured | |||||||
| Baseline | 892 | 228 (51.8) | 350 (77.4) | ||||
| Follow‐up | 1059 | 333 (74.0) | 403 (66.2) | 0.1271 (0.0487) | 1.21 (0.71 to 2.08) | 0.4813 | |
| Smoking status recorded | |||||||
| Baseline | 1045 | 168 (38.0) | 197 (32.7) | ||||
| Follow‐up | 1059 | 421 (93.6) | 273 (44.8) | 0.0773 (0.0333) | 33.96 (14.49 to 79.62) | <0.0001 | |
| BMI measured or weight checked | |||||||
| Baseline | 1059 | 189 (42.2) | 220 (36.0) | ||||
| Follow‐up | 1059 | 396 (88.2) | 281 (46.1) | 0.0125 (0.0412) | 10.14 (4.99 to 20.55) | <0.0001 | |
| Risk factor management | |||||||
| BP<140/85 mm Hg | |||||||
| Baseline | 1004 | 184 (43.1) | 246 (42.6) | ||||
| Follow‐up | 962 | 250 (56.1) | 223 (43.2) | 0.0002 (0.0034) | 1.61 (1.22 to 2.13) | 0.0113 | |
| Cholesterol<5 mmol/l | |||||||
| Baseline | 785 | 202 (60.5) | 255 (56.5) | ||||
| Follow‐up | 735 | 249 (74.3) | 254 (63.5) | 0.1115 (0.0214) | 1.58 (1.05 to 2.37) | 0.0314 | |
| Prescribing of secondary prevention medicines | |||||||
| Lipid‐lowering medication | |||||||
| Baseline | 1080 | 230 (49.9) | 280 (45.2) | ||||
| Follow‐up | 1080 | 275 (59.6) | 322 (52.0) | 0.0051 (0.0079) | 1.99 (1.06 to 3.74) | 0.0281 | |
| Aspirin | |||||||
| Baseline | 1080 | 300 (65.1) | 388 (62.7) | ||||
| Follow‐up | 1080 | 314 (68.1) | 411 (66.4) | 0.0021 (0.0165) | 1.08 (0.84 to 1.40) | 0.5502 | |
| Beta‐blocker† | |||||||
| Baseline | 586 | 110 (44.2) | 122 (36.2) | ||||
| Follow‐up | 586 | 125 (50.2) | 141 (41.8) | 0.0001 (0.0024) | 1.43 (1.19 to 1.99) | 0.0354 | |
| ACE inhibitor† | |||||||
| Baseline | 489 | 84 (39.4) | 117 (42.4) | ||||
| Follow‐up | 489 | 103 (48.4) | 140 (50.7) | 0.0179 (0.0194) | 0.97 (0.68 to 1.43) | 0.9301 | |
Values are numbers (percentages) unless stated otherwise.
BMI, body mass index; BP, blood pressure.
*Values adjusted for baseline, age, gender and practice. †Patients with past history of myocardial infarction only.
Table 3 Differences between cardiovascular risk factors at 12 month follow‐up in intervention and control group patients with CHD at 12 month follow‐up.
| Outcome measure | Number of observations | Intervention (n = 461) | Control (n = 691) | Mean difference* (95% CI) | p Value |
|---|---|---|---|---|---|
| Systolic BP (mm Hg) | 962 | 134.72 (0.86) | 139.30 (0.80) | 4.58 (2.28 to 6.88) | 0.0015 |
| Diastolic BP (mm Hg) | 962 | 75.18 (0.46) | 78.71 (0.43) | 3.53 (2.29 to 4.78) | 0.0003 |
| Total cholesterol (mmol/l) | 735 | 4.53 (0.05) | 4.71(0.43) | 0.18 (0.05 to 0.30) | 0.0123 |
| BMI (kg/m2) | 392 | 29.30 (0.21) | 29.53 (0.22) | 0.25 (−0.35 to 0.86) | 0.1491 |
Values are adjusted means (SE).
BMI, body mass index; BP, blood pressure.
*Adjusted for baseline, age, gender, smoking status and cluster effect.
Heart failure
Of the 362 patients with presumed CHF, 34.8% (126/362) had a previously confirmed diagnosis of left ventricular systolic dysfunction at baseline: 34.7% (51/147) in the intervention group and 34.9% (75/215) in the control group. In patients with a confirmed diagnosis of left ventricular systolic dysfunction, 73/126 (58%) were already being prescribed ACE inhibitors at baseline. While no statistically significant differences were observed between groups, in both groups the proportion of patients being treated with inhibitors of the renin‐angiotensin‐aldosterone system and beta‐blockers increased over the study period (table 4).
Table 4 Number of patients with confirmed left ventricular systolic dysfunction prescribed appropriate therapy.
| Number of observations | Number (%) | OR (95% CI) | p Value | ||
|---|---|---|---|---|---|
| Intervention (n = 51) | Control (n = 75) | ||||
| ACE inhibitor | |||||
| Baseline | 126 | 33 (64.7) | 40 (53.3) | 0.41 (0.21 to 1.37) | 0.1531 |
| Follow‐up | 126 | 33 (64.7) | 51 (68.0) | ||
| ACE or ARB* | |||||
| Baseline | 126 | 39 (76.5) | 50 (66.7) | 0.57 (0.14 to 2.32) | 0.4312 |
| Follow‐up | 126 | 43 (84.3) | 62 (82.7) | ||
| Beta‐blocker | |||||
| Baseline | 126 | 14 (27.5) | 24 (32.0) | 1.72 (0.25 to 11.82) | 0.5802 |
| Follow‐up | 126 | 20 (39.2) | 28 (37.3) | ||
| Carvedilol or bisoprolol | |||||
| Baseline | 126 | 9 (17.6) | 14 (18.7) | 2.75 (0.63 to 11.86) | 0.1721 |
| Follow‐up | 126 | 17 (33.3) | 18 (24.0) | ||
Values are number (percentage).
*Angiotensin receptor blocker.
Confirmation or exclusion of diagnosis of left ventricular systolic dysfunction
Of those people with a presumed but previously unconfirmed diagnosis of CHF, 36.5% (35/96) in the intervention group underwent echocardiographic examination, compared to 10% (14/140) in the control group (odds ratio (OR): 5.64, 95% confidence interval (CI): 2.81 to 11.31, p<0.01) during the 12‐month follow‐up period. Potential confounding covariates age and gender were included in the mixed model. Left ventricular systolic dysfunction was confirmed by echocardiography in 19/96 (19.8%) and 7/140 (5.0%) people with presumed but unconfirmed heart failure in the intervention and control groups, respectively (adjusted OR: 4.69, 95% CI: 1.88 to 11.66, p<0.01). In addition, 16.7% (16/96) in the intervention group and 5.0% (7/140) in the control group had a diagnosis of left ventricular systolic dysfunction excluded (adjusted OR: 3.80, 95% CI: 1.50 to 9.64, p<0.01). In patients with a past history of myocardial infarction, 9.6% (14/146) in the intervention group and 3.8% (6/156) in the control group had a new diagnosis of left ventricular systolic dysfunction made by echocardiogrphy during the follow‐up year (adjusted OR: 2.65, 95% CI: 0.99 to 7.09, p = 0.05).
Quality‐of‐life measurements
Tables 5 and 6 show the overall adjusted average scores at baseline and the estimated quality‐of‐life scores at follow‐up by treatment groups in patients with CHD and left ventricular systolic dysfunction, respectively. In patients with CHD (table 5), there were significant differences in SF‐36 follow‐up scores for physical functioning, general health, vitality, social functioning and mental health. Seattle Angina Questionnaire scores in intervention group patients with angina were significantly better for intervention group patients compared to controls for exertional capacity, and there were also borderline differences for angina frequency and quality of life. There were no significant differences in any of the SF‐36 health status domains or the LVD‐36 scores in patients with a confirmed diagnosis of left ventricular systolic dysfunction when comparing patients in the two study groups at follow‐up (table 6).
Table 5 Quality‐of‐life scores after 12 month follow‐up for patients with CHD.
| Adjusted mean score* | p Value‡ | ||||
|---|---|---|---|---|---|
| Intervention | Control | ||||
| SF‐36 Domain | |||||
| Physical functioning | 50.79 | 45.46 | 0.0220 | ||
| Role physical | 40.16 | 36.13 | 0.1889 | ||
| Bodily pain | 58.60 | 55.59 | 0.3941 | ||
| General health | 49.22 | 46.66 | 0.0141 | ||
| Vitality | 48.54 | 43.01 | 0.0001 | ||
| Social functioning | 70.27 | 62.51 | 0.0002 | ||
| Role emotional | 56.75 | 51.11 | 0.2341 | ||
| Mental health | 71.63 | 67.14 | 0.0012 | ||
| Seattle Angina Questionnaire† | |||||
| Exertional capacity | 49.38 | 44.13 | 0.0014 | ||
| Angina stability | 60.66 | 58.29 | 0.2513 | ||
| Angina frequency | 76.97 | 74.10 | 0.0452 | ||
| Treatment satisfaction | 87.24 | 84.79 | 0.3705 | ||
| Quality of life | 66.38 | 62.43 | 0.0571 | ||
Higher scores indicate better quality of life for both questionnaires.
*Adjusted for cluster effect and baseline scores, and where appropriate for age and gender. †Angina patients only. ‡p Values based on robust estimates of standard errors.
Table 6 Quality‐of‐life scores after 12 month follow‐up for patients with confirmed diagnosis of left ventricular systolic dysfunction.
| Adjusted mean score* | p Value† | ||
|---|---|---|---|
| Intervention | Control | ||
| SF‐36 Domain | |||
| Physical functioning | 39.32 | 36.95 | 0.6221 |
| Role physical | 20.01 | 20.81 | 0.9973 |
| Bodily pain | 54.81 | 55.13 | 0.9061 |
| General health | 45.04 | 40.12 | 0.3937 |
| Vitality | 40.52 | 40.08 | 0.9144 |
| Social functioning | 56.93 | 58.77 | 0.7282 |
| Role emotional | 35.97 | 39.46 | 0.6721 |
| Mental health | 66.23 | 64.74 | 0.7740 |
| LVD‐36 Score | 48.19 | 50.63 | 0.6683 |
Higher SF‐36 and lower LVD‐36 scores indicate better quality of life.
*Adjusted for cluster effect and baseline scores, and where appropriate for age and gender. †p Values based on robust estimates of standard errors.
Discussion
This study indicates that a nurse‐led disease management programme in primary care can lead to improvement in quality of care, including secondary prevention, for patients with CHD and CHF. The intervention also led to improvements in referral for echocardiography, resulting in more complete clarification of the presence or absence of left ventricular systolic dysfunction in patients with presumed CHF.
Strengths and limitations of the study
The limitations and difficulties of conducting this type of pragmatic, interventional trial in primary care are well recognised.6,30 It is a limitation of this study that we recruited practices from one locality. While only 43% of eligible patients participated in the study, this is not particularly low for such trials conducted in primary care.31 The recruitment rate was higher for the control group than for the intervention group and for ethical reasons we were unable to compare the characteristics of patients who took part in the study and those who did not. As appointments with the specialist nurse were offered only to patients in the intervention group, it was not possible to blind those invited to participate in terms of the group to which their practice had been allocated. It is therefore possible that the different recruitment procedures for the two groups led to differences in motivation. However, intervention and control group patients who agreed to participate appeared reasonably well matched overall in terms of data collected at baseline. As data collectors were required to extract data relating to intervention group patients' appointments with the specialist nurse, it was also not possible for those collecting data to be blinded to practice group allocation. Although the components of a successful intervention seem to be regular contact with patients, education and optimisation of treatment,32,33 it is difficult to determine from this trial which facet or facets of a complex, multifactorial intervention led to improvements in care.
The study has a number of strengths. First, and importantly, it has widespread generalisability as the sample size was large and the intervention pragmatically designed for a primary care setting. Second, our study was well placed to assess the true impact of the intervention due to the inclusion of a control group. The trial was clustered by practice to reduce the risk of contamination. Finally, there were few exclusion criteria and we used validated generic and disease‐specific self‐administered quality‐of‐life questionnaires. The baseline characteristics of the cohorts were reasonably well balanced and we controlled for differences in our analysis. Of those patients consenting to participate, very few withdrew from the study.
Comparison with literature
A review of trials of nurse‐led secondary prevention clinics34 identified four relevant randomised trials, of which three were conducted in primary care in the UK. This review highlighted differences in the findings from these trials in terms of impact on factors such as lifestyle, drug treatment, blood pressure and lipid control, and quality of life. However, the overall conclusion was that these clinics have potential for improving both medical and lifestyle aspects of secondary prevention with a resulting impact on quality of life and mortality. In one of the trials included in the review, Moher et al.6 used a composite outcome measure of assessment of three risk factors, whereas our trial showed absolute improvements specifically in blood pressure control and lipid management, with a relatively low NNT to achieve either of these hard outcomes. The SHIP trial, not included in the review, assessed secondary prevention care of patients with newly diagnosed CHD, but failed to show any improvements in health outcomes.35 However, in this trial the specialist nurses did not provide clinical care but coordinated care between primary and secondary care after hospital discharge.
A recent, large, disease management programme for patients with confirmed CHF showed significant survival benefits in patients with symptomatic systolic CHF.36 In our study, benefits were demonstrated in terms of patients in the intervention group being more likely than controls to have a diagnosis of left ventricular systolic dysfunction confirmed or excluded. With regard to the management of patients with confirmed diagnosis of left ventricular systolic dysfunction, the intervention in our trial was less effective than we had anticipated. This may be in part due to the relatively high provision of appropriate secondary prevention therapies at baseline, compared to reports in recent studies. In our study sample, over two‐thirds of patients with left ventricular systolic dysfunction were being treated with an ACE inhibitor at recruitment, compared to previously reported rates of 40%37 and 55%.38 With regard to quality‐of‐life measures, our study showed beneficial effects in patients with CHD but no change for those with CHF. Some previous studies of disease management programmes in CHF have also failed to show any impact on quality‐of‐life measures,39,40 while other studies have shown equivocal results in this context.41,42
Interpretation of findings
A disease management programme can lead to improvements in the care of patients with CHD and CHF in primary care. These improvements could lead to an increase in meeting targets set by the Quality and Outcomes Framework, which was introduced in 2004 to determine payments to UK general practices based on quality of care and which currently includes evidence‐based process and outcome indicators relating to the management of patients with CHD and heart failure. Our trial was pragmatic and could easily be widely implemented in the primary care setting. Disease management programmes are likely to be most beneficial in those settings where usual care is suboptimal43 and for many of the outcomes we assessed, existing management was better than previously reported. This suggests that we had underestimated the secular trends in prescribing of secondary prevention drugs and prescriptions of CHF medication, with a likely reduction in the overall effect of the intervention. Nevertheless, quality of care in the management of patients with CHF includes accurate diagnosis of the type of CHF. Our trial found that a disease management programme significantly improved the proportion of patients previously labelled as having CHF who had confirmation or exclusion of a diagnosis of left ventricular systolic dysfunction. Larger trials are required to assess the effect of specialist nurses in primary care on the management of patients with left ventricular systolic dysfunction.
Acknowledgements
We wish to acknowledge the contribution made by the participating general practices.
Abbreviations
CHD - coronary heart disease
CHF - chronic heart failure
NNT - numbers needed to treat
Footnotes
Funding: The study was funded by the Trent NHS Executive, UK.
Competing interests: KK, IS and AF have received sponsorship for attending conferences and small honoraria from pharmaceutical companies that make beta‐blockers, ACE inhibitors and angiotensin receptor blockers. JB and LG have received sponsorship for attending conferences from these companies.
References
- 1.Sans S, Kesteloot H, Kromhout D. The burden of cardiovascular diseases mortality in Europe. Task Force of the European Society of Cardiology on Cardiovascular Mortality and Morbidity Statistics in Europe. Eur Heart J 1997181231–1248. [PubMed] [Google Scholar]
- 2.Unal B, Critchley J A, Capewell S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation 20041091101–1107. [DOI] [PubMed] [Google Scholar]
- 3.Ergin A, Muntner P, Sherwin R.et al Secular trends in cardiovascular disease mortality, incidence, and case fatality rates in adults in the United States. Am J Med 2004117219–227. [DOI] [PubMed] [Google Scholar]
- 4.EUROSPIRE II Study Group Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries; principal results from EUROASPIRE II Euro Heart Survey Programme. Eur Heart J 200122554–572. [DOI] [PubMed] [Google Scholar]
- 5.Campbell N C, Thain J, Deans H G.et al Secondary prevention in coronary heart disease: baseline survey of provision in general practice. BMJ 19983161430–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher M, Yudkin P, Wright L.et al Cluster randomised controlled trial to compare three methods of promoting secondary prevention of coronary heart disease in primary care. BMJ 20013221338–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMurray J J, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart (British Cardiac Society) 200083596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart S, MacIntyre K, Hole D J.et al More ‘malignant' than cancer? Five‐year survival following a first admission for heart failure. Eur J Heart Fail 20013315–322. [DOI] [PubMed] [Google Scholar]
- 9.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 10.Flather M D, Yusuf S, Kober L.et al Long‐term ACE‐inhibitor therapy in patients with heart failure or left‐ventricular dysfunction: a systematic overview of data from individual patients. ACE‐Inhibitor Myocardial Infarction Collaborative Group. Lancet 20003551575–1581. [DOI] [PubMed] [Google Scholar]
- 11.Clarke K W, Gray D, Hampton J R. Evidence of inadequate investigation and treatment of patients with heart failure. British Heart Journal 199471584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuat A, Hungin A P, Murphy J J. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ 2003326196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khunti K, Hearnshaw H, Baker R.et al Heart failure in primary care: qualitative study of current management and perceived obstacles to evidence‐based diagnosis and management by general practitioners. Eur J Heart Fail 20024771–777. [DOI] [PubMed] [Google Scholar]
- 14.McAlister F A, Lawson F M E, Teo K K.et al Randomised trials of secondary prevention programmes in coronary heart disease: Systematic review. BMJ 2001323957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whellan D J M. Metaanalysis and review of heart failure disease management randomized controlled clinical trials. Am Heart J 2005149722–729. [DOI] [PubMed] [Google Scholar]
- 16.Gonseth J, Guallar‐Castillon P, Banegas J R.et al The effectiveness of disease management programmes in reducing hospital re‐admission in older patients with heart failure: a systematic review and meta‐analysis of published reports. Eur Heart J 2004251570–1595. [DOI] [PubMed] [Google Scholar]
- 17.Roccaforte R, Demers C, Baldassarre F.et al Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta‐analysis. Eur J Heart Fail 200571133–1144. [DOI] [PubMed] [Google Scholar]
- 18.Bruggink‐Andre de la Porte P W, Lok D J, van Wijngaarden J.et al Heart failure programmes in countries with a primary care‐based health care system. Are additional trials necessary? Design of the DEAL‐HF study. Eur J Heart Fail 20057910–920. [DOI] [PubMed] [Google Scholar]
- 19.Davies M K, Hobbs F D R, Davis R C.et al Prevalence of left‐ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: A population based study. Lancet 2001358439–444. [DOI] [PubMed] [Google Scholar]
- 20.Khunti K, Baker R, Grimshaw G. Diagnosis of patients with chronic heart failure in primary care: usefulness of history, examination, and investigations. British Journal of General Practice 20005050–54. [PMC free article] [PubMed] [Google Scholar]
- 21.Wood D, Durrington P, Poulter N.et al Joint British recommendations on prevention of coronary heart disease in clinical practice. Heart (British Cardiac Society) 199880(Suppl 2)S1–29. [PMC free article] [PubMed] [Google Scholar]
- 22.Anonymous Prevention of coronary heart disease in clinical practice. Recommendations of the Second Joint Task Force of European and other Societies on coronary prevention. Eur Heart J 1998191434–1503. [DOI] [PubMed] [Google Scholar]
- 23.Scottish Intercollegiate Guidelines Network Diagnosis and treatment of heart failure due to left ventricular systolic dysfunction. Report No. 35. Edinburgh: SIGN 1999
- 24.Swedberg K, Cleland J, Dargie H.et al Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005261115–1140. [DOI] [PubMed] [Google Scholar]
- 25.Garratt A M, Ruta D A, Abdalla M I.et al The SF36 health survey questionnaire: an outcome measure suitable for routine use within the NHS? BMJ 19933061440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spertus J A, Winder J A, Dewhurst T A.et al Development and evaluation of the Seattle Angina Questionnaire: A new functional status measure for coronary artery disease. Journal of the American College of Cardiology 199525333–341. [DOI] [PubMed] [Google Scholar]
- 27.O'Leary C J, Jones P W. The left ventricular dysfunction questionnaire (LVD‐36): Reliability, validity, and responsiveness. Heart 200083634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feder G, Griffiths C, Eldridge S.et al Effect of postal prompts to patients and general practitioners on the quality of primary care after a coronary event (POST): randomised controlled trial. BMJ 19993181522–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell N C, Ritchie L D, Thain J.et al Secondary prevention in coronary heart disease: a randomised trial of nurse led clinics in primary care. Heart 199880447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson S, Delaney B C, Roalfe A.et al Randomised controlled trials in primary care: case study. BMJ 200032124–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutten F H, Moons K G, Cramer M J.et al Recognising heart failure in elderly patients with stable chronic obstructive pulmonary disease in primary care: cross sectional diagnostic study. BMJ 20053311379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blue L, Lang E, McMurray J J V.et al Randomised controlled trial of specialist nurse intervention in heart failure. BMJ 2001323715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McAlister F A, Teo K K, Taher M.et al Insights into the contemporary epidemiology and outpatient management of congestive heart failure. Am Heart J 199913887–94. [DOI] [PubMed] [Google Scholar]
- 34.Campbell N C. Secondary prevention clinics: improving quality of life and outcome. Heart. 2004;90(Suppl IV)29–32. [DOI] [PMC free article] [PubMed]
- 35.Jolly K, Bradley F, Sharp S.et al Randomised controlled trial of follow up care in general practice of patients with myocardial infarction and angina: final results of the Southampton heart integrated care project (SHIP). The SHIP Collaborative Group. BMJ 1999318706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galbreath A D, Krasuski R A, Smith B.et al Long‐term healthcare and cost outcomes of disease management in a large, randomized, community‐based population with heart failure. Circulation 20041103518–3526. [DOI] [PubMed] [Google Scholar]
- 37.McAlister F A, Murphy N F, Simpson C R.et al Influence of socioeconomic deprivation on the primary care burden and treatment of patients with a diagnosis of heart failure in general practice in Scotland: population based study. BMJ 20043281110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komajda M, Follath F, Swedberg K.et al The EuroHeart Failure survey programme: A survey on the quality of care among patients with heart failure in Europe. Part 2: Treatment. Eur Heart J 200324464–474. [DOI] [PubMed] [Google Scholar]
- 39.Ekman I, Andersson B, Ehnfors M.et al Feasibility of a nurse‐monitored, outpatient‐care programme for elderly patients with moderate‐to‐severe, chronic heart failure. Eur Heart J 1998191254–1260. [DOI] [PubMed] [Google Scholar]
- 40.Mejhert M, Kahan T, Persson H.et al Limited long term effects of a management programme for heart failure. Heart 2004901010–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doughty R N, Wright S P, Pearl A.et al Randomized, controlled trial of integrated heart failure management: The Auckland Heart Failure Management Study. Eur Heart J 200223139–146. [DOI] [PubMed] [Google Scholar]
- 42.Stromberg A, Martensson J, Fridlund B.et al Nurse‐led heart failure clinics in Sweden. Eur J Heart Fail 20013139–144. [DOI] [PubMed] [Google Scholar]
- 43.Krumholz H M M, AHA/ACC Conference Evaluating quality of care for patients with heart failure. Circulation 2000101e122–e140. [DOI] [PubMed] [Google Scholar]