Abstract
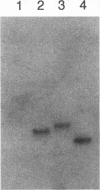
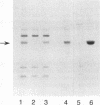
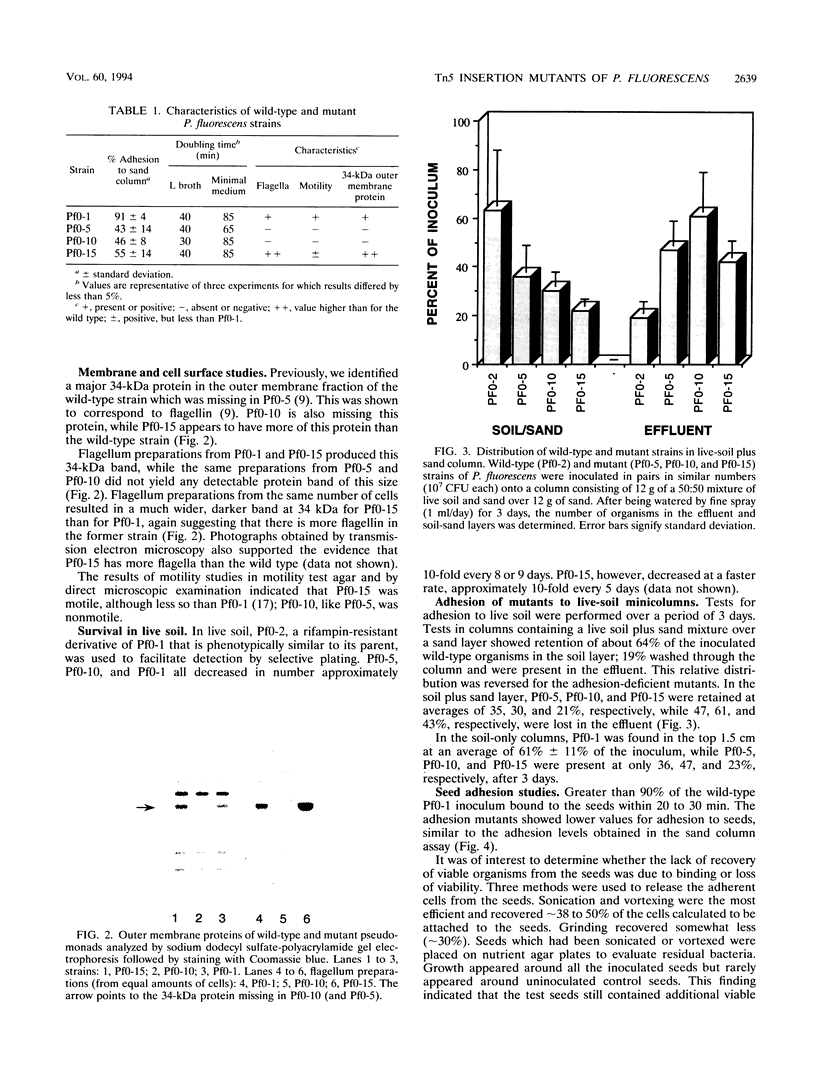
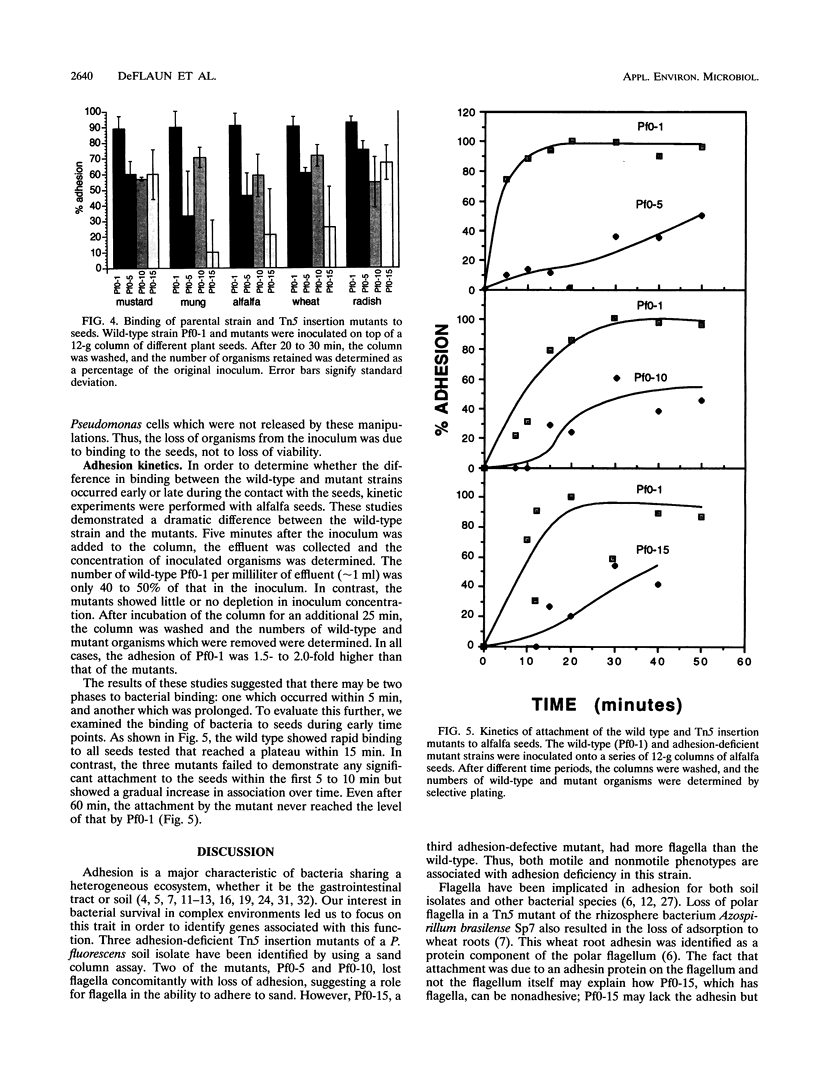
Tn5 insertion mutants of a soil isolate, Pseudomonas fluorescens Pf0-1, were selected for decreased ability to adhere to quartz sand in a column assay. Three adhesion-deficient mutants that differed in the location of the Tn5 insertion in the chromosome were isolated and compared with the wild-type strain. One mutant, Pf0-5, was described previously as an adhesion-defective, nonmobile, flagellumless mutant (M. F. DeFlaun, A. S. Tanzer, A. L. McAteer, B. Marshall, and S. B. Levy, Appl. Environ. Microbiol. 56:112-119, 1990). Another insertion mutant, Pf0-10, was also missing flagella and the 34-kDa outer membrane protein that was absent in Pf0-5 but present in the wild-type strain. The third mutant (Pf0-15) had increased amounts of this 34-kDa outer membrane protein and more flagella than the wild-type strain. These mutants also displayed decreased ability to adhere to sterile and natural (live) soil and to a variety of plant seeds. In kinetics studies, the wild-type strain showed an initial rapid binding to seeds followed by a later slow phase of binding. The mutant strains were defective in the initial stages of attachment but did show the later slow binding. The findings indicate that the same mutations that affect binding to sand and soil also affect adhesion to plant seeds.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cowan M. M., Taylor K. G., Doyle R. J. Kinetic analysis of Streptococcus sanguis adhesion to artificial pellicle. J Dent Res. 1986 Oct;65(10):1278–1283. doi: 10.1177/00220345860650101501. [DOI] [PubMed] [Google Scholar]
- Crews J. L., Colby S., Matthysse A. G. Agrobacterium rhizogenes mutants that fail to bind to plant cells. J Bacteriol. 1990 Nov;172(11):6182–6188. doi: 10.1128/jb.172.11.6182-6188.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croes C., Van Bastelaere E., DeClercq E., Eyers M., Vanderleyden J., Michiels K. Identification and mapping of loci involved in motility, adsorption to wheat roots, colony morphology, and growth in minimal medium on the Azospirillum brasilense Sp7 90-MDa plasmid. Plasmid. 1991 Sep;26(2):83–93. doi: 10.1016/0147-619x(91)90048-2. [DOI] [PubMed] [Google Scholar]
- De Mot R., Proost P., Van Damme J., Vanderleyden J. Homology of the root adhesin of Pseudomonas fluorescens OE 28.3 with porin F of P. aeruginosa and P. syringae. Mol Gen Genet. 1992 Feb;231(3):489–493. doi: 10.1007/BF00292721. [DOI] [PubMed] [Google Scholar]
- De Weger L. A., van der Vlugt C. I., Wijfjes A. H., Bakker P. A., Schippers B., Lugtenberg B. Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J Bacteriol. 1987 Jun;169(6):2769–2773. doi: 10.1128/jb.169.6.2769-2773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deflaun M. F., Tanzer A. S., McAteer A. L., Marshall B., Levy S. B. Development of an Adhesion Assay and Characterization of an Adhesion-Deficient Mutant of Pseudomonas fluorescens. Appl Environ Microbiol. 1990 Jan;56(1):112–119. doi: 10.1128/aem.56.1.112-119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Staneloni R. J., Rubin R. A., Nester E. W. Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol. 1985 Mar;161(3):850–860. doi: 10.1128/jb.161.3.850-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Geis G., Leying H., Suerbaum S., Mai U., Opferkuch W. Ultrastructure and chemical analysis of Campylobacter pylori flagella. J Clin Microbiol. 1989 Mar;27(3):436–441. doi: 10.1128/jcm.27.3.436-441.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain A. K., Alexander M. Enhancing Soybean Rhizosphere Colonization by Rhizobium japonicum. Appl Environ Microbiol. 1984 Sep;48(3):468–472. doi: 10.1128/aem.48.3.468-472.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Lund B., Lindberg F., Normark S. Structure and antigenic properties of the tip-located P pilus proteins of uropathogenic Escherichia coli. J Bacteriol. 1988 Apr;170(4):1887–1894. doi: 10.1128/jb.170.4.1887-1894.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. H., Savage D. C. Purification and characterization of flagella from Roseburia cecicola, an obligately anaerobic bacterium. J Gen Microbiol. 1985 Aug;131(8):2075–2078. doi: 10.1099/00221287-131-8-2075. [DOI] [PubMed] [Google Scholar]
- Maslow J. N., Mulligan M. E., Adams K. S., Justis J. C., Arbeit R. D. Bacterial adhesins and host factors: role in the development and outcome of Escherichia coli bacteremia. Clin Infect Dis. 1993 Jul;17(1):89–97. doi: 10.1093/clinids/17.1.89. [DOI] [PubMed] [Google Scholar]
- O'Sullivan D. J., O'Gara F. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev. 1992 Dec;56(4):662–676. doi: 10.1128/mr.56.4.662-676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J. P., Idziak E. S. Role of flagella in adhesion of Pseudomonas fluorescens to tendon slices. Appl Environ Microbiol. 1991 Jun;57(6):1635–1639. doi: 10.1128/aem.57.6.1635-1639.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Weller D. M. Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol. 1988 Aug;170(8):3499–3508. doi: 10.1128/jb.170.8.3499-3508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper S. J. Production of Pili (Fimbriae) by Pseudomonas fluorescens and Correlation with Attachment to Corn Roots. Appl Environ Microbiol. 1987 Jul;53(7):1397–1405. doi: 10.1128/aem.53.7.1397-1405.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Zehnder A. J. Influence of interfaces on microbial activity. Microbiol Rev. 1990 Mar;54(1):75–87. doi: 10.1128/mr.54.1.75-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]