Abstract
The therapeutic benefits of fungal β-glucans have been demonstrated as immuno-stimulating agents. In this study, we aimed to explore the mechanisms used by yeast β-glucan-rich particles to activate murine resident macrophages for cytokine secretion. We demonstrated that resident macrophages were effectively activated by whole yeast β-glucan particles (WGPs), such as with the up-regulation of co-stimulatory molecules and the secretion of cytokines. The binding ability of WGPs and the levels of cytokine secretion in resident macrophages were significantly inhibited by soluble yeast β-glucan but not by blockade of zymosan glucan receptor dectin-1. In addition, WGP-stimulated cytokine secretion was partially dependent on the MyD-88 pathway but was not significantly affected in CR3-deficient (CR3−/−) mice. Furthermore, we showed that Syk kinase was recruited upon WGP stimulation and was required for the production of cytokines. Taken together, these observations suggest that β-glucan recognition is necessary but not sufficient to induce inflammatory response on resident macrophages. In addition, β-glucan particles may use differential mechanisms for cytokine secretion in resident macrophages that may modulate both innate and adaptive immunity.
Keywords: Macrophage, cytokine, pattern recognition receptor, β-glucan, Toll-like receptor
Introduction
β-Glucans, potent biological response modifiers (BRMs) derived from yeast, fungi, grains, and seaweed have shown therapeutic benefits in a variety of animal disease models [1-7]. However, the mechanism of action of β-glucans has not been fully elucidated. Previous studies demonstrated that soluble small molecular mass yeast β-glucans function through macrophages, neutrophils, and NK cells to prime complement receptor 3 (CR3, CD11b/CD18, Mac-1, αMβ2 integrin) for killing iC3b-opsonized yeast or tumor cells [8-12]. On the other hand, the whole yeast β-glucan particles (WGPs) are predominately taken up and processed by macrophages to release active fragments, which subsequently prime neutrophils for CR3-dependent cellular cytotoxicity (CR3-DCC) [6, 13] or bind to hematopoietic progenitor cells (HPC) for the enhancement of complement-mediated hematopoietic recovery [14]. Interestingly, the binding of WGPs to macrophages is CR3-indepdendent. On the contrary, dectin-1, a C-type lectin receptor, was previously defined as a major yeast cell wall zymosan β-glucan receptor [15, 16]. It has been shown that a complex of toll-like receptor (TLR)-2 and/or TLR6 in collaboration with dectin-1 receptor is required for the production of proinflammatory cytokines such as TNF-α in response to zymosan stimulation [17-19]. These studies are particularly important to understand fungus-host interaction. Furthermore, recent data from dectin-1 knockout mice highlighted the importance of β-glucan receptors in the fungal infection [20, 21].
Zymosan is a stimulatory cell wall extract of Saccharomyces cerevisiae primarily containing β-glucans as well as other components such as mannans, mannoproteins, and chitin [22]. Among the cell wall components, β-glucan comprises approximately 12% to 14% of the dry cell weight of yeast. In contrast, particular yeast β-glucan WGPs are the further purified β-glucan spheres from the same strain of yeast cells based on alkali solubility [13]. Alkaline extractions dissolve the yeast cytoplasmic material, cell-wall mannans and proteins, and a minor glucan component leaving intact WGPs. WGP preparation yields a hollow, β-glucan sphere, roughly the same size (2-4 microns) as the original yeast cell and containing >75% β-glucan. The focus of this study is to determine whether different preparations of yeast particular β-glucan elicit their specific biological functions, e.g., cytokine secretion via differential mechanisms.
Macrophages are professional phagocytes that act as the first line of defense provided by the innate immune system. Resident macrophages are widely distributed in tissues and are one of the primary cell types to sense and respond to microbial invaders. Spleen tyrosine kinase (Syk) is a 72-kDa protein kinase belonging to the Syk-ZAP 70 family [23]. The Syk tyrosine kinase is essential for integrin signaling in neutrophils, macrophages, and platelets [24]. As a key regulator in intracellular signaling pathway, Syk plays important roles in both innate and adaptive immunity [25]. Studies have demonstrated that Syk kinase was required for zymosan-stimulated IL-10 and IL-2 production by murine dendritic cells (DCs) [26]. Therefore, it is critical to explore whether Syk kinase regulates macrophage function upon WGP β-glucan stimulation.
In the present study, we explore the activation mechanisms of resident macrophages with WGP β-glucan stimulation. We show that murine resident macrophages were able to bind WGP, resulting in the up-regulation of accessory molecules and MHC class II and the secretion of proinflammatory cytokines. As compared to zymosan, WGPs are less potent for the stimulation of cytokine production. These events appear to be dependent, at least partially, on β-glucan recognition and the MyD-88 pathway as well as on the recruitment of Syk phosphorylation. However, they are independent of CR3 or dectin-1. These data suggest different preparations of particulate β-glucans may use differential mechanisms to activate resident macrophages that may impact both innate and adaptive immunity.
Materials and Methods
Mice and reagents
C57Bl/6 wildtype (WT) mice were purchased from National Cancer Institute (Frederick, MD). C57Bl/6 CR3−/− mice were a gift from Dr. Tanya Mayadas-Norton (Brigham& Women's Hospital and Harvard Medical School, Boston, MA). C57Bl/6 MyD88-deficient (MyD88−/−) mice were purchased from Oriental Yeast Company (Tokyo, Japan). All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Louisville.
Anti-mouse mAbs CD40-FITC, CD69-FITC, CD80-FITC, CD86-FITC, MHC II-FITC, F4/80-PE and appropriately labeled isotype controls were purchased from BD Pharmingen (San Diego, CA). Anti-mouse cytokine mAbs IL-4-PE, IL-6-PE, IL-10-PE, IL-12-PE, IFN-γ-PE, and TNF-α-PE and isotype-PE were purchased from eBioscience (San Diego, CA). Anti-mouse dectin-1 mAb (2A11) was purchased from Cell Sciences (Canton, MA). Syk kinase inhibitor piceatannol and mannan were purchased from Sigma (St. Louis, MO).
Preparation of β-glucans
WGP (Biothera, Eagan, MN) were purified from the cell wall of Saccharomyces cerevisiae through a series of alkaline and acid extractions to yield hollow yeast cell wall “ghosts” composed primarily of long polymers of β-1,3 glucose with 3-6% of the backbone glucose units possessing a β(1,6) branch [13]. WGP were hydrated by adding distilled water and were sonicated to produce a single-particle suspension. For some studies, WGP were labeled with fluorescein dichlorotriazine (DTAF; Molecular Probes-Invitrogen, Carlsbad, CA), which covalently reacts with hydroxyl groups of polysaccharides using a modification of the procedures suggested by the manufacturer. To remove any trace amounts of LPS contamination, the WGP were suspended in 200 mM NaOH for 20 min at room temperature (RT), washed thoroughly and re-suspended in LPS-free water as described previously [6]. β-Glucan poly-(1,6)-β-D-glucopyranosyl-(1,3)-β-D-glucopyranose (PGG) (Biothera, Eagan, MN) is a soluble, pharmaceutical-grade β-glucan compound derived from a same strain of yeast as WGPs. It is a triple helical molecule with an average molecular mass of 150-kDa. F1-5 and F5-14 are also soluble β-glucans with an average molecular mass of 239-kDa and 95-kDa, respectively. Zymosan was purchased from Sigma (St. Louis, MO).
Binding assays and cytokine measurement
Freshly isolated resident peritoneal macrophages or thioglycollate-elicited peritoneal macrophages were washed twice in ice-cold PBS. Cells were plated at indicated numbers in 24-well plates in RPMI-1640 medium overnight and washed three times with PBS before each assay. For the binding assay, a total of 5×105 peritoneal cells were placed in plastic tubes. Inhibitors (PGG-β-glucan, 2A11 dectin-1 mAb, mannan or irrelevant IgG2b mAbs, 100 μg/ml) were added and incubated on ice for 30 min before the addition of DTAF-labeled or non-labeled WGPs (MOI=10:1) according to different experiments. Cells were incubated on ice for 1 hr, washed, and fixed with BD Cytofix buffer. Macrophages were identified by F4/80 expression. The percentage of WGP binding macrophages and mean fluorescent intensity (MFI) were determined by flow cytometry analysis. Images were acquired using immunofluorescence microscopy (Nikon Eclipse TE300 confocal cell images).
For cytokine measurement, macrophages (5 × 105) were stimulated with WGP (100 μg/ml) at 37 °C for 3 hrs in the presence or absence of different inhibitors, including PGG β-glucan, 2A11, mannan, or Syk kinase inhibitor piceatannol. Supernatants were harvested for cytokine measurement. Cytokines were measured using the anti-mouse ELISA MAXTM SET Deluxe kits (Biolegend, San Diego, CA) as described by the manufacturer. BDTM Cytometric Bead Array (CBA) mouse inflammation kits were also used to measure the cytokine secretion.
Detection of Syk phosphorylation by immunofluorescence microscopy
Resident peritoneal macrophages placed on glass bottom Willco-well dishes (World Precision Instruments, Sarasota, FL) were fed DTAF-labeled WGPs for 30 min in the presence of 10 mM sodium orthovanadate. Cells were then fixed and permeabilized with BD Cytofix and Perm/wash buffer. Phosphorylated Syk was detected with anti-phospho-Syk Ab (Cell Signaling Technology, Danvers, MA) followed by mouse anti-rabbit-IgG-PE (Jackson Immunoresearch, West Grove, PA). Fluorescent microscopy images were acquired as described above.
Western blot
The whole macrophage lysates before or after WGP stimulation were used for western blot to detect Syk and phospho-Syk with anti-Syk or anti-phospho-Syk Abs (Cell Signaling Technology). VisualizerTM Western blot detection kit (Upstate, Lake Placid, NY) and the ECL kit (Amersham, Pittsburgh, PA) were used according to the manufacture's instructions.
Flow cytometry
For intracellular cytokine staining, macrophages were stimulated with 100 μg/ml WGP for 3 hrs. Cells were then washed with PBS and detached with trypsin/EDTA buffer. The cells were incubated with anti-CD32/CD16 mAb to block Fc receptors, fixed, and then permeabilized. Cells were stained for 1hr with PE-labeled indicated anti-cytokine mAbs or isotype mAb. For intracellular phosphorylated Syk staining, secondary goat anti-rabbit-IgG-FITC Ab (eBioscience, San Diego, CA) was followed after anti-phospho-Syk Ab staining. The cells were then collected with a FACS Calibur cytometer (BD Immunocytometry Systems, San Jose, CA) and analyzed using FlowJo (Tree Star, Ashland, OR).
Graphing and statistical analysis of data
Data from experiments were entered into Prism 4.0 (Graph Pad Software, San Diego, CA) to generate graphs to determine the significance of differences between data sets. Student's t test and two-way Anova were used to compare significance between different groups.
Results
Up-regulation of surface accessory molecules and MHC class II on macrophages upon exposure to particulate yeast β-glucan WGP
Previous studies showed that exposure of macrophages to different microbial products induces differential activation status and modulates surface receptor expression and co-stimulation [27]. To evaluate the expression levels of activation markers and molecules critical for Ag presentation, membrane surface markers on resident peritoneal macrophages were analyzed before and after WGP stimulation. As shown in Figure 1, CD40, CD80, CD86 and MHC class II molecules were constitutively expressed on resident peritoneal macrophages, while CD69 was not detected. In response to WGP stimulation, CD40, CD80, CD86, and MHC class II molecules were significantly upregulated. In addition, CD69, also known as an activation inducer molecule, was expressed on resident peritoneal macrophages after stimulation. Thus, particulate yeast β-glucan WGPs could stimulate resident macrophages to upregulate critical accessory and MHC class II molecules, which are important for Ag presentation.
Figure 1. Surface marker expression on murine resident peritoneal macrophages.
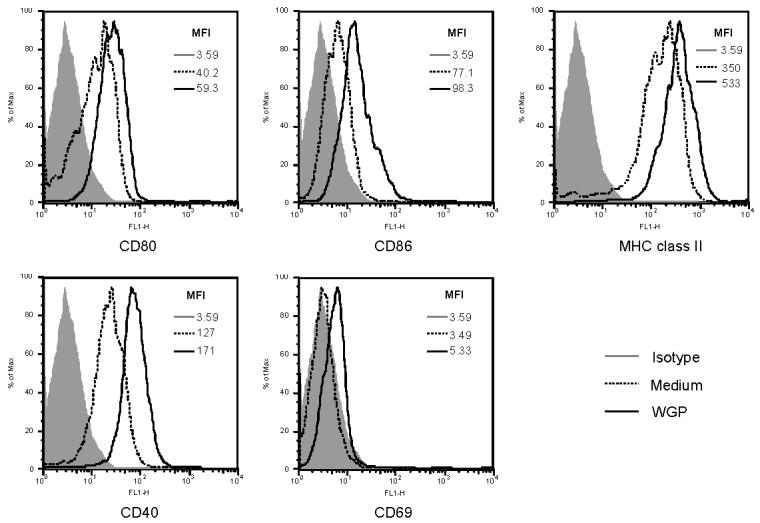
Resident peritoneal macrophages from C57Bl/6 WT mice were collected and stimulated with WGP β-glucan (100 μg/ml) for 12 hrs. The cells were harvested and stained with indicated mAbs or isotype controls. Unstimulated macrophages were used as controls. The histograms are one representative of three independent experiments.
Particulate β-glucan, but not soluble β-glucan, stimulates resident macrophages to secrete cytokines
Next, we examined whether WGPs could stimulate resident macrophages for cytokine production. To that end, WGP-stimulated murine resident peritoneal macrophages were analyzed for a panel of cytokine production using intracellular cytokine staining assessed by flow cytometry. As shown in Figure 2A, resident peritoneal macrophages were able to secret IL-6 and TNF-α, which were essential cytokines required for the successful control of many pathogens, while IL-4, IL-10, IL-12, and IFN-γ were not detected (Figure 2A and data not shown).
Figure 2. Cytokine secretion by resident peritoneal macrophages upon WGP or zymosan stimulation.
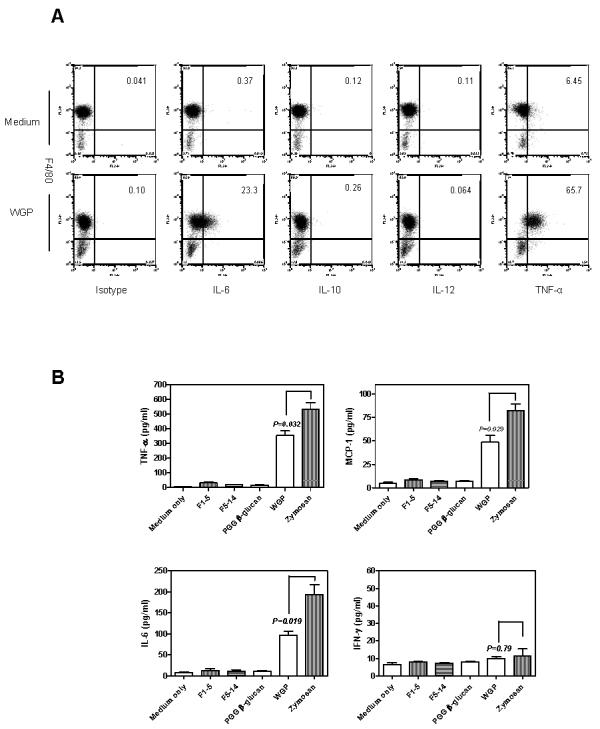
(A) C57Bl/6 WT resident peritoneal macrophages (5×105) were stimulated for 3 hrs in the presence or absence of WGPs (100 μg/ml) before adding BD GolgiPLUG. The cells were cultured for an additional 3 hrs, then harvested and stained with mAb F4/80-PE-Cy5.5. After washing, cells were fixed, permeabilized, and stained with the indicated anti-cytokine mAbs or isotype control. (B) Resident macrophages (5×105) were stimulated with WGPs, F1-5, F5-14, or PGG β-glucan (100 μg/ml) or zymosan (100 μg/ml) for 3 hrs. The supernatants were collected and detected for cytokines using BD Cytometric Bead Array (CBA) kit. Mean ± SE of duplicates are shown. Data represent one representative of two independent experiments.
To further understand if the secretion of cytokines is mediated exclusively by β-glucans, preparations of different molecular masses of pure soluble β-glucans derived from the same strain of yeast were used as stimuli side by side with WGPs. None of the pure soluble β-glucans were able to mediate the tested cytokine production (IL-10 and IL-12 data not shown), while particulate WGP β-glucan was able to stimulate the TNF-α, IL-6 and MCP-1 production in resident macrophages (Fig. 2B). In addition, the levels of cytokine secretion upon zymosan stimulation were significantly higher than those in response to WGP treatment.
Soluble PGG β-glucan inhibits resident macrophage binding and cytokine secretion by WGP
To investigate whether cytokines induced by WGPs were indeed mediated through β-glucan recognition, we performed the cytokine inhibition assay using soluble PGG β-glucan or soluble mannan. As observed in Figure 3A and 3B (upper panel), PGG β-glucan, but not mannan, was able to significantly block the binding of WGPs to resident macrophages. In addition, the levels of IL-6 and TNF-α were significantly reduced in the presence of soluble PGG β-glucan (Fig. 3B, lower panel). However, soluble PGG β-glucan does not trigger cytokine production, suggesting that β-glucan recognition is necessary but not sufficient for particulate β-glucan WGPs to activate resident macrophages for cytokine secretion.
Figure 3. Cytokine secretion and binding of WGP by resident macrophages requires β-glucan recognition.
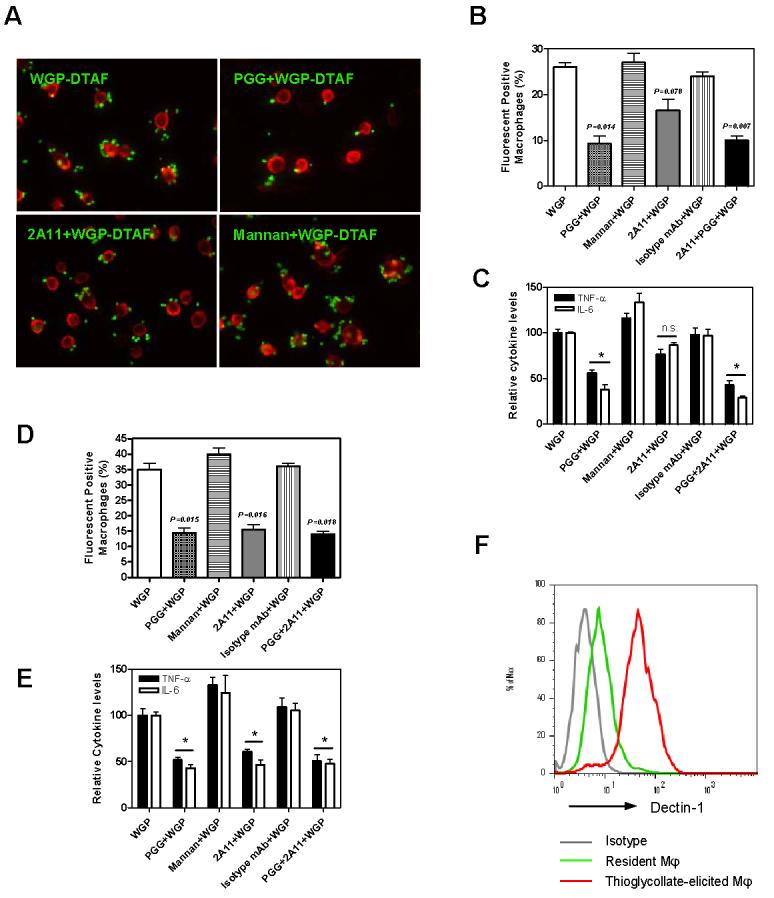
(A) Fluorescent microscope showed resident peritoneal macrophages (red) plated on glass bottom Willco-dishes were able to bind to WGPs (green). The WGP binding was significantly inhibited by PGG β-glucan, but not by dectin-1 mAb 2A11 or mannan. Original magnification: ×200. (B) Flow cytometry analysis showed significant inhibition of WGP binding by PGG soluble β-glucan (p=0.014) but not by mAb 2A11 (p=0.078) or mannan (upper panel). (C) PGG β-glucan, but not mAb 2A11 or mannan (n.s., no significance), significantly blocked the cytokine secretion by WGP (*P<0.05). The levels of TNF-α and IL-6 secretion mediated by WGPs are arbitrarily setup as 100 (relative cytokine level). (D) Both PGG β-glucan and 2A11 significantly blocked the WGP binding on thioglycollate-elicited macrophages (*P<0.05). Mean ± SE of triplicates is shown. (E). Both PGG β-glucan and anti-dectin-1 mAb 2A11 significantly inhibited the cytokine secretion in response to WGPs on thioglycollate-elicited macrophages. The levels of TNF-α and IL-6 production mediated by WGPs are arbitrarily setup as 100 (relative cytokine level). (F) Dectin-1 expression levels on activated macrophages were much higher than that on resident macrophages. Data represent one representative of three independent experiments.
Dectin-1 has been extensively studied as a zymosan β-glucan receptor [17, 28-30]. It was shown that zymosan stimulated murine macrophages to secrete cytokines such as TNF-α via the dectin-1 dependent pathway. However, TNF-α production was not affected in dectin-1-deficient thioglycollate-elicited macrophages [20]. We next examined whether the WGP binding and cytokine secretion were also dependent on dectin-1. Although anti-dectin-1 mAb (2A11) was titrated from 200 μg/ml to 25 μg/ml (data now shown), none of them showed significant inhibition (Fig. 3A, B and C). In contrast, anti-dectin-1 mAb was able to inhibit the cytokine secretion of resident macrophage stimulated by zymosan (data not shown), suggesting the component and structural differences between WGPs and zymosan.
Further experiments using thioglycollate-elicited macrophages indicated that both PGG β-glucan and 2A11 mAb significantly inhibited the binding and cytokine secretion by WGPs (Fig. 3D and E), which led us to examine the expression levels of dectin-1 on different activation states of macrophages. As shown in Figure 3F, the expression level of dectin-1 was much higher on thioglycollate-elicited macrophages than that on resident macrophages.
Secretion of IL-6 and TNF-α by resident peritoneal macrophages in response to WGP stimulation is partially dependent on MyD88 pathway
To investigate whether particulate yeast β-glucan WGPs activate resident peritoneal macrophages to produce cytokines through CR3, we compared the binding and cytokine secretion levels of resident peritoneal macrophages from C57Bl/6 WT or CR3−/− mice. Despite the non-opsonic binding of WGP to macrophages in CR3−/− mice showing lower MFI compared to that in WT mice, the differences were not significant (Fig. 4A). In addition, no significant inhibition was observed in cytokine production (Fig. 4B). To explore the possible pathways that may be involved in WGP-stimulated macrophage cytokine secretion, we examined the TLR signaling pathway. TLRs are PRRs used by innate immune cells to detect the presence of a variety of pathogens. Although the TLR signaling pathway differs for various TLRs, most TLRs begin with the adaptor protein MyD88. As indicated in Figure 4B, production of both IL-6 and TNF-α by resident macrophages was significantly decreased in MyD88−/− macrophages.
Figure 4. The secretion of cytokines IL-6 and TNF-α by resident macrophages is dependent on the MyD88 pathway.
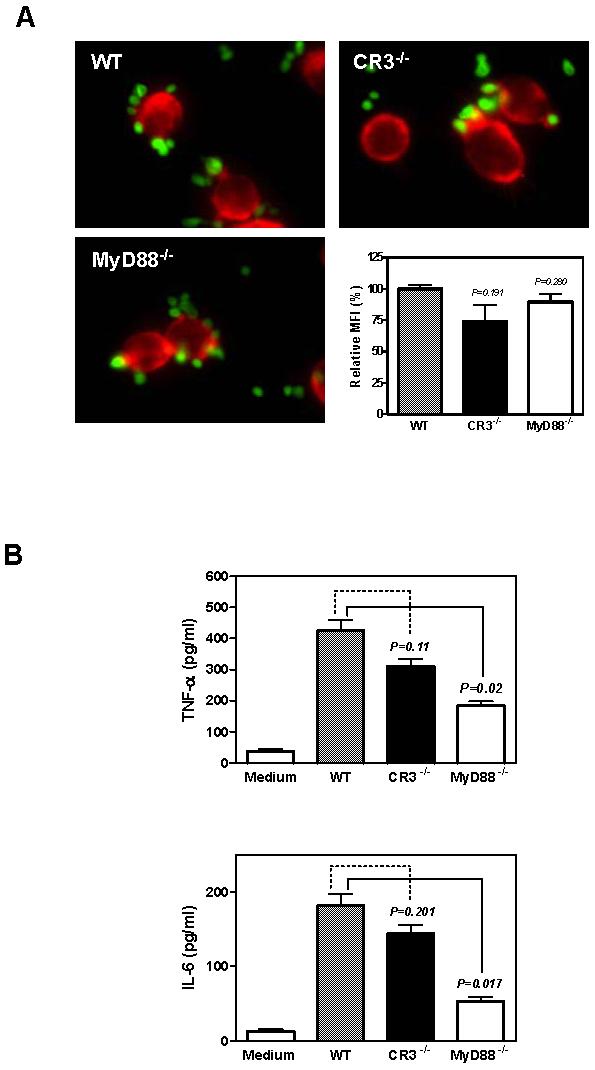
(A) WGP (green) binding of resident macrophages (red) from WT, CR3−/−, and MyD88−/− mice showed no significant difference. Original magnification: ×400. (B) The secretion of cytokine IL-6 and TNF-α by resident macrophages from Myd88−/− mice decreased dramatically while the cytokine level of resident macrophages from CR3−/− decreased slightly compared to that from WT mice. Mean ± SE of triplicates are shown. Data represent one representative set of two independent experiments.
WGP stimulation activates macrophage Syk kinase
Syk kinase, in concert with the related kinase ZAP-70, was a key regulator of phagocytic cell activation and played a critical role in immunoreceptor and/or non-immunoreceptor signaling [25]. To assess if Syk kinase was activated in macrophages after exposure to WGPs, resident peritoneal macrophages were stimulated and analyzed for Syk activation, e.g., Syk-phosphorylation. As determined by western blot, similar levels of Syk kinase were observed in both non-stimulated and WGP-stimulated resident peritoneal macrophages. Augmented Syk phosphorylation was detected in WGP-stimulated macrophages (Fig. 5A). Intracellular staining with anti-phospho-Syks further demonstrated that Syk kinase was phosphorylated in a subset of macrophages (Fig. 5B) although almost all of the macrophages internalized WGP. These data were reminiscent of Syk kinase activation mediated by dectin-1 as a dynamic subset of macrophages activated for the production of reactive oxygen species (ROS) [31]. To determine whether WGP stimulation directly recruits Syk kinase, fluorescent labeled WGP were co-cultured with resident peritoneal macrophages. Confocal microscopy showed that WGP particles were absorbed by the macrophages and co-localized with phosphorylated Syk (Fig. 5C). Notably, most of these phagosomes showed clear phospho-Syk accumulation in the WGP stimulated macrophages. In addition, Syk phosphorylation was significantly inhibited by soluble PGG β-glucan (Fig. 5D), suggesting that Syk kinase activation was intermediated by β-glucan recognition.
Figure 5. Syk phosphorylation in resident peritoneal macrophages stimulated by WGP.
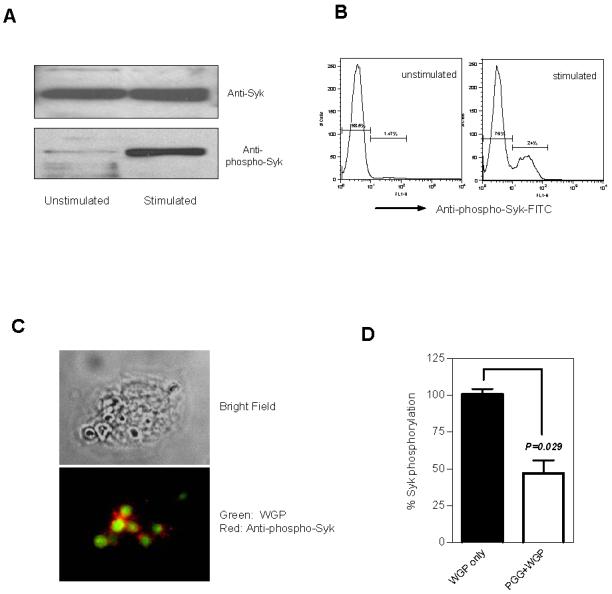
Resident peritoneal macrophages were co-cultured with WGPs (100 μg/ml) for 30 min in the presence of sodium orthovanadate. The cells were collected, lysed, and total Syk and phosphorylation of Syk were determined by (A) western blot using anti-Syk Ab or anti-phospho-Syk Ab (Tyr352). Unstimulated macrophages were served as a control. Syk phosphorylation of macrophages stimulated with or without WGPs was also detected by intracellular staining using anti-phospho-Syk Ab assessed by flow cytometry (B). Data suggest that Syk kinase was phosphorylated in ∼24% of resident peritoneal macrophages upon WGP stimulation. Syk kinase phosphorylated was determined by immunofluorescence microscopy (C). Macrophages were stimulated with DTAF-WPGs (green) and phosphorylated Syk was determined by anti-phospho-Syk-PE mAb (red). Original magnification: ×600. (D) Resident peritoneal macrophages were pre-cultured with or without soluble PGG β-glucan (200 μg/ml). The cells were then stimulated with WGP (100 μg/ml) and stained with anti-phopho-Syk Ab for intracellular phopho-Syk expression assessed by flow cytometry. The percent of phospho-Syk positive macrophages from the WGP-treated group was arbitrarily set up as 100%. Data suggest that soluble PGG β-glucan inhibits WGP-stimulated Syk kinase phosphorylation.
Syk kinase activation is required for WGP-induced IL-6 and TNF-α production
Since WGP stimulation induced macrophage Syk kinase phosphorylation, we next explored the role of Syk kinase in WGP recognition and cytokine secretion. Piceatannol, a selective inhibitor of protein tyrosine kinase Syk, was used to block the activity of Syk [19]. The inhibitor was titrated so the used concentration would not have adverse effects on resident macrophage viability and binding ability. As indicated in Figure 6A, inhibition of Syk kinase with piceatannol at 50 μM did not affect macrophage-mediated WGP binding, suggesting the binding of WGPs was independent of Syk kinase. However, the level of IL-6 and TNF-α production by resident peritoneal macrophages was significantly decreased after treatment with piceatannol (Fig. 6B). Thus, our data suggest that Syk kinase is not required for WGP binding, but is required for the WGP-induced IL-6 and TNF-α production.
Figure 6. Syk phosphorylation is required for cytokine production by macrophages.
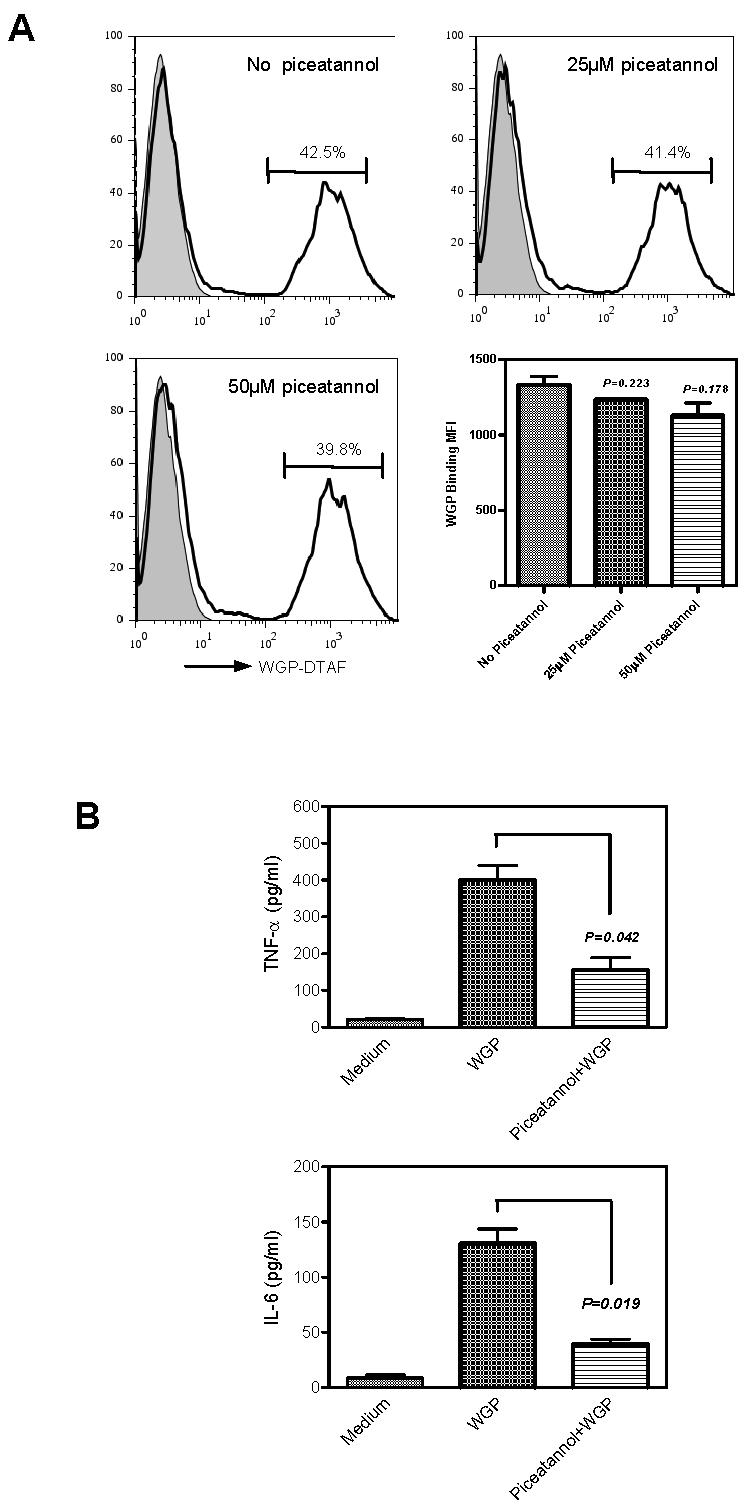
(A) Resident peritoneal macrophages were co-cultured with fluorescein-labeled WGP-DTAF in the presence of varying amounts of Syk inhibitor piceatannol. Up to 50 μM of piceatannol showed no adverse effects on WGP binding to resident peritoneal macrophages. (B) Resident peritoneal macrophages were cultured with WGPs (100 μg/ml) for 4 hrs in the presence or absence of piceatannol (50 μM). The levels of TNF-α and IL-6 in the cultural supernatants were determined with ELISA kits. The levels of TNF-α or IL-6 from macrophages treated with or without piceatannol were compared. Mean ± SE of triplicates are shown. Data are one representative set of three independent experiments.
Discussion
In this paper, we showed that particulate yeast β-glucan WGPs efficiently bound to resident peritoneal macrophages, which sequentially activated resident macrophages to upregulate expression of co-stimulatory molecules and MHC class II molecules, to recruit Syk kinase, and to secrete pro-inflammatory cytokine IL-6 and TNF-α. The cytokine secretion was blocked by soluble β-glucan, and was partially dependent on MyD88 pathway and Syk kinase phosphorylation.
Macrophages possess a variety of pattern recognition receptors (PRRs) that interact with highly conserved structures present on microorganisms such as fungi [32]. The engagement of these PRRs activates anti-microbial functions critical to innate immunity. Many PRRs related to β-glucan recognition on macrophages have been identified, namely CR3 [33], dectin-1 [15, 16], scavenger receptor [34], and lactosylceramide [35]. In the present study, we showed the WGP binding ability was not significantly decreased in CR3−/− resident macrophages, demonstrating that CR3 may not be important in β-glucan particle-mediated binding. Furthermore, the addition of anti-dectin-1 mAb did not significantly inhibit the WGP binding. Previous study suggests that SIGN-related 1 (SIGNR1) was a mannan-inhibitable receptor for zymosan on the surface of resident macrophages [36]. However, mannan was not able to inhibit the binding of WGPs to macrophages (Fig. 3), indicating that other innate receptors may be putatively involved in this particulate yeast β-glucan binding to resident peritoneal macrophages.
We found WGPs were able to stimulate resident macrophages to secrete pro-inflammatory cytokines, such as IL-6 and TNF-α. The cytokine production was a MyD88- and Syk-dependent process, however CR3 and dectin-1 did not play a leading role in these resident macrophages. Furthermore, β-glucan recognition was required as soluble PGG β-glucan significantly inhibited cytokine secretion. Although soluble yeast β-glucan did not directly stimulate resident macrophages for cytokine secretion (Fig 3B and 3C), it is possible that the β-glucans themselves may not induce the inflammatory response but that recognition of β-glucans in conjunction with other “danger triggers” such as TLR agonists stimulate this response. This notion is supported by previous studies with yeast cell wall zymosan demonstrating that dectin-1 and TLR-2 were synergized in zymosan-mediated TNF-α and IL-12 production [18]. In our study, we confirmed the MyD88 signaling pathway was required for WGP-mediated cytokine secretion, which was also confirmed by Pneumocystis carinii cell wall glucans [37] . However, anti-dectin-1 mAb was not able to significantly inhibit IL-6 and TNF-α production (Fig. 3C). It further supported the notion that β-glucan-rich particle WGPs may use different PRRs than dectin-1 or CR3 for binding and cytokine secretion on resident macrophages. Interestingly, dectin-1-deficient bone marrow-derived DCs produced normal levels of IL-10 and IL-12 in response to zymosan [20, 21]. This indicates that, at least in DCs, the involvement of other receptors other than dectin-1 is required in the recognition and response to zymosan.
These observed differences in the spectrum of cytokine secretion and involvement of different β-glucan receptors could be attributed to differential compositions between zymosan and WGPs. WGPs are insoluble particles, purified from the cell walls of a common form of yeast, Saccharomyces cerevisiae. WGPs mainly contain β-glucan (>75%) with long polymers of β(1,3) glucose and 3-6% of the backbone glucose units possessing a β(1,6) branch [13]. Zymosan is also a insoluble cell wall preparation from same strain of yeast but is one composed of fewer β-glucans (12%-14%) [22]. It is worth noting that while both zymosan and WGP stimulate macrophage for TNF-α production, zymosan induced far greater levels of TNF-α than WGP (Fig. 2B). It is likely zymosan contains more TLR ligands that synergize with dectin-1 for cytokine secretion. These data may also suggest that although β-glucan recognition is necessary, TLR signaling determines the levels of cytokine secretion. Another contributing factor is the activation states of macrophages. In the current study, we used resident peritoneal macrophages without any treatment prior to WGP stimulation, while most other studies used thioglycollate- or biogel-elicited macrophage or bone marrow-derived macrophages [17, 18]. Indeed, thioglycollate-elicited macrophages showed much higher dectin-1 expression compared to resident macrophages and dectin-1 mAb 2A11 was able to block the cytokine secretion by WGPs, indicating that dectin-1 is the major receptor for particulate β-glucans on stimulated macrophages rather than on resident macrophages. This notion was also proved in a study where GM-CSF and other cytokines were able to induce high dectin-1 expression on macrophages which in turn significantly affected zymosan binding and TNF-α production [38].
We further demonstrated Syk kinase was quickly phosphorylated after WGP stimulation and that Syk kinase was also required for the WGP-mediated IL-6 and TNF-α production. Previous study demonstrated that Syk kinase was required for zymosan-mediated IL-10 and IL-2 production by murine DCs [26]. Recent study also showed that β-glucan derived from Candida albicans induces human neutrophil migration through Src family kinase [39]. Consistent with these findings, the production of IL-6 was shown to be down-regulated in respiratory epithelial cells treated with Syk small interfering RNA or Syk inhibitor piceatannol, suggesting the potential role of Syk kinase in the regulation of IL-6 secretion [40]. Together with the previous reports, we therefore propose a mechanism of WGP-stimulated cytokine secretion occurring from resident peritoneal macrophages. First, particulate WGP β-glucans are bound to macrophages through different PRRs. The major recognition is mediated through β-glucans. Subsequently, WGP binding macrophages recruit Syk kinase upon phosphorylation. In addition, other components from WGP also engage TLRs via the MyD88 pathway. Finally, the activation of Syk kinase and TLR signaling leads to TNF-α and IL-6 production. Future work will require characterization of the involved PRRs for WGP recognition on resident macrophages and its interplay with TLRs for cytokine production.
Acknowledgements
The authors thank Drs. Robert Stout and Vaclav Vetvicka for critical reading of the manuscript.
Abbreviations
- BRM
biological response modifier
- CR3-DCC
CR3 dependent cellular cytotoxicity
- DTAF
dichlorotriazine
- HPC
hematopoietic progenitor cells
- MFI
mean fluorescent intensity
- TLR
Toll-like receptor
- PGG
poly-(1,6)-β-D-glucopyranosyl-(1,3)-β-D-glucopyranose
- PRR
pattern recognition receptor
- ROS
reactive oxygen species
- SIGNRI
SIGN-related 1
- Syk
spleen tyrosine kinase
- WGP
whole glucan particles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was supported by research funding from NIH RO1 CA86412 and the Kentucky Lung Cancer Research Board. JY is a recipient of American College of Rheumatology and Arthritis Foundation Investigator Award.
References
- 1.Di Luzio NR, Williams DL, McNamee RB, Edwards BF, Kitahama A. Comparative tumor-inhibitory and anti-bacterial activity of soluble and particulate glucan. Int J Cancer. 1979;24:773–779. doi: 10.1002/ijc.2910240613. [DOI] [PubMed] [Google Scholar]
- 2.Williams DL, Browder IW, Di Luzio NR. Immunotherapeutic modification of Escherichia coli--induced experimental peritonitis and bacteremia by glucan. Surgery. 1983;93:448–454. [PubMed] [Google Scholar]
- 3.Tsikitis VL, Albina JE, Reichner JS. Beta-glucan affects leukocyte navigation in a complex chemotactic gradient. Surgery. 2004;136:384–389. doi: 10.1016/j.surg.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Kournikakis B, Mandeville R, Brousseau P, Ostroff G. Anthrax-protective effects of yeast beta 1,3 glucans. MedGenMed. 2003;5:1. [PubMed] [Google Scholar]
- 5.Cheung NK, Modak S. Oral (1-->3),(1-->4)-beta-D-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma. Clin Cancer Res. 2002;8:1217–1223. [PubMed] [Google Scholar]
- 6.Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, Xing PX, Cheung NK, Ross GD. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 7.Wei D, Zhang L, Williams DL, Browder IW. Glucan stimulates human dermal fibroblast collagen biosynthesis through a nuclear factor-1 dependent mechanism. Wound Repair Regen. 2002;10:161–168. doi: 10.1046/j.1524-475x.2002.10804.x. [DOI] [PubMed] [Google Scholar]
- 8.Vetvicka V, Thornton BP, Ross GD. Soluble beta-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the beta-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 10.Xia Y, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. J Immunol. 1999;162:2281–2290. [PubMed] [Google Scholar]
- 11.Yan J, Vetvicka V, Xia Y, Coxon A, Carroll MC, Mayadas TN, Ross GD. Beta-glucan, a “specific” biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18) J Immunol. 1999;163:3045–3052. [PubMed] [Google Scholar]
- 12.Hong F, Hansen RD, Yan J, Allendorf DJ, Baran JT, Ostroff GR, Ross GD. Beta-glucan functions as an adjuvant for monoclonal antibody immunotherapy by recruiting tumoricidal granulocytes as killer cells. Cancer Res. 2003;63:9023–9031. [PubMed] [Google Scholar]
- 13.Yan J, Allendorf DJ, Brandley B. Yeast whole glucan particle (WGP) beta-glucan in conjunction with antitumour monoclonal antibodies to treat cancer. Expert Opin Biol Ther. 2005;5:691–702. doi: 10.1517/14712598.5.5.691. [DOI] [PubMed] [Google Scholar]
- 14.Cramer DE, Allendorf DJ, Baran JT, Hansen R, Marroquin J, Li B, Ratajczak J, Ratajczak MZ, Yan J. {beta}-Glucan enhances complement-mediated hematopoietic recovery after bone marrow injury. Blood. 2006;107:835–840. doi: 10.1182/blood-2005-07-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 16.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herre J, Marshall AS, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V, Reis e Sousa C, Gordon S, Brown GD. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004;104:4038–4045. doi: 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]
- 20.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 21.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Carlo FJ, Fiore JV. On the composition of zymosan. Science. 1958;127:756–757. doi: 10.1126/science.127.3301.756-a. [DOI] [PubMed] [Google Scholar]
- 23.Turner M, Schweighoffer E, Colucci F, Di Santo JP, Tybulewicz VL. Tyrosine kinase SYK: essential functions for immunoreceptor signalling. Immunol Today. 2000;21:148–154. doi: 10.1016/s0167-5699(99)01574-1. [DOI] [PubMed] [Google Scholar]
- 24.Mocsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26:208–214. doi: 10.1016/j.it.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis ESC. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 28.Brown GD, Gordon S. Fungal beta-glucans and mammalian immunity. Immunity. 2003;19:311–315. doi: 10.1016/s1074-7613(03)00233-4. [DOI] [PubMed] [Google Scholar]
- 29.Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. Embo J. 2005;24:1277–1286. doi: 10.1038/sj.emboj.7600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown GD, Gordon S. Immune recognition of fungal beta-glucans. Cell Microbiol. 2005;7:471–479. doi: 10.1111/j.1462-5822.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 31.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106:2543–2550. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 33.Ross GD. Regulation of the adhesion versus cytotoxic functions of the Mac-1/CR3/alphaMbeta2-integrin glycoprotein. Crit Rev Immunol. 2000;20:197–222. [PubMed] [Google Scholar]
- 34.Rice PJ, Kelley JL, Kogan G, Ensley HE, Kalbfleisch JH, Browder IW, Williams DL. Human monocyte scavenger receptors are pattern recognition receptors for (1-->3)-beta-D-glucans. J Leukoc Biol. 2002;72:140–146. [PubMed] [Google Scholar]
- 35.Zimmerman JW, Lindermuth J, Fish PA, Palace GP, Stevenson TT, DeMong DE. A novel carbohydrate-glycosphingolipid interaction between a beta-(1-3)-glucan immunomodulator, PGG-glucan, and lactosylceramide of human leukocytes. J Biol Chem. 1998;273:22014–22020. doi: 10.1074/jbc.273.34.22014. [DOI] [PubMed] [Google Scholar]
- 36.Taylor PR, Brown GD, Herre J, Williams DL, Willment JA, Gordon S. The role of SIGNR1 and the beta-glucan receptor (dectin-1) in the nonopsonic recognition of yeast by specific macrophages. J Immunol. 2004;172:1157–1162. doi: 10.4049/jimmunol.172.2.1157. [DOI] [PubMed] [Google Scholar]
- 37.Lebron F, Vassallo R, Puri V, Limper AH. Pneumocystis carinii cell wall beta-glucans initiate macrophage inflammatory responses through NF-kappaB activation. J Biol Chem. 2003;278:25001–25008. doi: 10.1074/jbc.M301426200. [DOI] [PubMed] [Google Scholar]
- 38.Willment JA, Lin HH, Reid DM, Taylor PR, Williams DL, Wong SY, Gordon S, Brown GD. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol. 2003;171:4569–4573. doi: 10.4049/jimmunol.171.9.4569. [DOI] [PubMed] [Google Scholar]
- 39.Sato T, Iwabuchi K, Nagaoka I, Adachi Y, Ohno N, Tamura H, Seyama K, Fukuchi Y, Nakayama H, Yoshizaki F, Takamori K, Ogawa H. Induction of human neutrophil chemotaxis by Candida albicans-derived beta-1,6-long glycoside side-chain-branched beta-glucan. J Leukoc Biol. 2006;80:204–211. doi: 10.1189/jlb.0106069. [DOI] [PubMed] [Google Scholar]
- 40.Ulanova M, Puttagunta L, Marcet-Palacios M, Duszyk M, Steinhoff U, Duta F, Kim MK, Indik ZK, Schreiber AD, Befus AD. Syk tyrosine kinase participates in beta1-integrin signaling and inflammatory responses in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L497–507. doi: 10.1152/ajplung.00246.2004. [DOI] [PubMed] [Google Scholar]


