Abstract
To evaluate humoral (antibody) and cell mediated immune (CMI) responses, 30 healthy young adults were either given inactivated influenza vaccine with or without QS21 adjuvant. Vaccination site pain and postvaccination myalgias were greater in the QS21 group. Serum antibody increases occurred in 73 to 93% of subjects for each vaccine and antigen at 2 weeks and 4 weeks but frequencies and mean titers for the two vaccines were not different. No differences in T cell cytotoxicity were detected for either vaccine for influenza A or B infected cells. IFN-γ for both vaccine groups was increased in supernates after 3 days but not 7 days of stimulation in the cytotoxicity tests; amounts for the two vaccines were similar. To further evaluate CMI, remaining PBMCs were stimulated overnight with cells infected with each vaccine strain; an increase in spot forming cells (sfc) for Granzyme B and IFN-γ was found for all subjects and in 51 of 54 sfc tests. A slightly higher response in the Gran B test for QS21 subjects was suggested, but no clear immune response advantage was identified among healthy adults for QS21 adjuvanted influenza vaccine.
Keywords: Influenza, influenza vaccine, humans, immune responses, antibody, cell-mediated immune responses, IFN-γ, Granzyme B
Introduction
Inactivated influenza virus vaccines (IVV), have been available and used for prevention of influenza and its complications for decades. During that period, viral antigens, dosages of the antigen and methods for antigen quantitation have varied (1,2). For about the past 25 years, vaccines distributed for use in humans have contained antigens from each of the current circulating strains standardized to contain 15 μg of the hemagglutinin (HA) of each strain suspended in a saline solution (aqueous vaccine). These vaccines induce serum anti-HA antibody in the majority of vaccinated persons although responses tend to be less common and of lower magnitude in the elderly (2).
There is general agreement that protection of the elderly with current inactivated vaccines is not optimal and that improvement in their efficacy is desirable. A number of options have been proposed for improving immune responses to current vaccines so as to improve their protective power; among these is addition of an adjuvant to the vaccine preparation (3). A variety of adjuvants are available; one of these is QS21, a purified saponin from the bark of the South American tree, Quillaja saponaria (4). QS21 adjuvant is known as a T helper 1 cell adjuvant that enhances both humoral and cell mediated immune responses (4-6). Preclinical studies by us in mice comparing aqueous IVV to IVV containing the adjuvants QS21, monophosphoryl lipid A (MPL), or incomplete Freund's adjuvant (IFA) revealed that QS21 was superior for inducing antibody responses and protection in both unprimed and primed mice (7). IFA was included because it was used extensively in humans as an adjuvant for IVV in the 1950s and was shown to induce significant levels of protection (8). It was inferior to QS21 as an adjuvant in our preclinical studies (7).
The present study was designed to determine if QS21 used as an adjuvant with standard aqueous trivalent IVV would induce greater serum antibody responses than standard vaccine in humans as it had done in the mouse model and whether cell mediated immune (CMI) responses would be induced and be greater among persons given the vaccine containing QS21 adjuvant.
Materials and Methods
Subjects
Subjects were healthy young adults between 18 and 40 years of age; sixteen were male and 14 female. Fifteen subjects were white, five were African-American, four were Hispanic, three were Asian, one was of American Indian/Alaskan native ancestry; the racial/ethnic group for two subjects was unknown. Each subject was screened for good health by completing a health history questionnaire and undergoing any physical examination indicated by the health history. Prior to vaccination, blood pressure, pulse, and oral temperature were documented as within the normal range: all subjects were free of any acute illness and all females had a negative urine test for pregnancy on the day of vaccination. The protocol was reviewed and approved by the Baylor College of Medicine Institutional Review Board for Human Research before commencing the trial.
Vaccines
The inactivated influenza vaccine was purchased in the open market. It consisted of the 2000 – 2001 formulation prepared by Aventis Pasteur (now Sanofi Pasteur) Inc. The vaccine strains were A/Panama/2007/99 (H3N2), A/New Caledonia/20/99 (H1N1) and B/Yamanashi/166/98 present as 15 μg of the HA of each strain. The experimental vaccines were prepared on site by addition of normal saline or QS21 adjuvant to the standard vaccine such that a 0.6 ml volume would contain the standard concentration of viral antigens with or without 50 μg of the QS21 adjuvant.
Nucleoprotein in the vaccine was measured in an ELISA assay. Briefly, microtiter plates were coated with monoclonal anti-influenza NP antibody (Serotec, Raleigh, NC) overnight at 4°C. After blocking, diluted (1:4 or 1:16) influenza vaccine was added and incubated at 37°C for 2 hours. For detection, an FITC-conjugated anti-influenza NP monoclonal antibody (Virostat, Portland, ME) was added and incubated at 37°C for 1 hour, followed by biotin-conjugated rat-anti-FITC monoclonal antibody (Serotec). After incubation with peroxidase-conjugated streptavidin, the assay was developed with an ABTS substrate kit for horseradish peroxidase (Zymed Laboratories, South San Francisco, CA). The O.D. was determined at a wavelength of 415 nm and the amount of NP in the vaccine obtained from a standard curve using rNP. The vaccine contained 22 μg of influenza A NP.
Design
Thirty healthy adults were randomly assigned to receive 0.6 ml IM of the standard aqueous vaccine (0.5 ml of standard vaccine plus 0.1 ml saline) or 0.6 ml of the QS21 adjuvanted vaccine (0.5 ml of standard vaccine plus 0.1 ml of QS21 adjuvant). Vaccines were administered by an unblinded person but all clinical and laboratory evaluations were performed by persons unaware of the vaccine given to the volunteer. Blood was obtained before and two and four weeks after vaccination for antibody and CMI assays.
All subjects were observed for thirty minutes and again one day after vaccination for adverse events. In addition, each subject completed a daily recording of symptoms and oral temperature for a period of seven days. Injection site (pain, redness, swelling) and systemic symptoms (fever, headache, malaise, nausea, or body ache) were graded on a severity scale from 0 to 3 (see Table 1 for description). Serious adverse events (SAEs) were defined as life-threatening adverse events, significant or persistent disability, hospitalization, or death.
Table 1.
Adverse Effects after Vaccination with Inactivated Influenza Virus Vaccine With or Without QS21 Adjuvant
| No. Subjects with Indicated Severity (0-3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Aqueous Vaccine | QS21 Vaccine | ||||||||
| Evaluation Time | Adverse Effect | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Day 0 (Observed) | Local Pain:1 | ||||||||
| Immediate | 14 | 1 | 0 | 0 | 13 | 2 | 0 | 0 | |
| At 30 min | 13 | 2 | 0 | 0 | 5 | 9 | 1 | 0 | |
| Day 1 (Observed) | Local: | ||||||||
| Pain1 | 7 | 8 | 0 | 0 | 2 | 9 | 4 | 0 | |
| Redness2 | 15 | 0 | 0 | 0 | 14 | 1 | 0 | 0 | |
| Swelling/induration2 | 15 | 0 | 0 | 0 | 14 | 1 | 0 | 0 | |
| Systemic:3 | |||||||||
| Fever | 15 | 0 | 0 | 0 | 14 | 15 | 0 | 0 | |
| Feverish | 15 | 0 | 0 | 0 | 13 | 2 | 0 | 0 | |
| Headache | 14 | 1 | 0 | 0 | 12 | 3 | 0 | 0 | |
| Fatigue | 14 | 1 | 0 | 0 | 10 | 5 | 0 | 0 | |
| Myalgias | 15 | 0 | 0 | 0 | 10 | 5 | 0 | 0 | |
| Days 1-74 (Diary) | Local: | ||||||||
| Pain1 | 7 | 4 | 4 | 0 | 1 | 3 | 11 | 0 | |
| Redness2 | 15 | 0 | 0 | 0 | 13 | 2 | 0 | 0 | |
| Swelling2 | 15 | 0 | 0 | 0 | 13 | 2 | 0 | 0 | |
| Systemic:3 | |||||||||
| Fever | 15 | 0 | 0 | 0 | 14 | 1 | 0 | 0 | |
| Headache | 10 | 4 | 1 | 0 | 9 | 5 | 1 | 0 | |
| Fatigue | 11 | 4 | 0 | 0 | 8 | 6 | 1 | 0 | |
| Myalgias | 15 | 0 | 0 | 0 | 6 | 6 | 3 | 0 | |
| Nausea/vomiting | 12 | 2 | 1 | 0 | 13 | 2 | 0 | 0 | |
0 = none, 1 = tender to touch, 2 = hurts to move, 3 = unable to move
0 = none, 1 = 0.5 – 5 cm, 2 = 5 – 10 cm, 3 = >10 cm
0 = none, 1 = slight/mild, 2 = interferes with activity, 3 = incapacitating
Highest severity for day 1-7 interval
Temp = 100.6°F
Serologic Assays
Serum obtained before and four weeks after vaccination was tested for antibody in hemagglutination-inhibition (HAI) and neutralization (neut) tests as described previously (9,10). Antigens for assays were the vaccine antigens A/Panama/2007/99 (H3N2), A/New Caledonia/20/99 (H1N1), and B/Yamanashi/166/98. Whole virus was used for the HAI antibody tests; concentrations of reagents were altered and an erythrocyte adsorption step was added to permit a starting dilution of 1:4. A four-fold or greater increase in HAI or neut titer from baseline to one month after immunization was considered significant.
CMI Assays
Peripheral blood mononuclear cells (PBMC) were obtained and cryopreserved as described previously (11). Viruses used in CMI assays were A/Taiwan/1/86 (H1N1), B/Panama/45/90 and the three vaccine strains.
Cytotoxicity
Assays for cytotoxic lymphocytes (CTLs) were conducted as described previously (11,12). Briefly, cryopreserved PBMCs were thawed, washed and verified as >95% viable. PBMCs for stimulation were infected with A/Taiwan/1/86 (H1N1) or B/Panama/45/90 virus and then co-cultured with responder PBMCs at a 1:5 stimulator:responder ratio for seven days at 37°C. Target cells for CTL assays were thawed and incubated overnight at 37°C; A/Taiwan or B/Panama virus and 51Cr were then added to the targets and incubated for two hours. Stimulated cells from day 7 (effector cells) were added to target cells at a varying effector:target cell ratio. The mixture was incubated in microtiter plates for four hours followed by determination of radioactivity (cpm) in a gamma counter. The percent specific cytolytic activity was calculated using a described formula (11). Lysis of ≥10% was considered significant and an increase from prevaccination levels of ≥5% at two or four weeks was considered to be a significant increase.
Cytokine ELISA
Cell culture supernates were obtained from the cytotoxicity assays on day 3 and again on day 7 of the in vitro stimulation and assayed for IFN-γ, IL-4, and TNF-α. Cytoscreen ELISA kits were used for IFN-γ and TNF-α; IL-4 was determined using Cytoscreen™ Ultra Sensitive ELISA kits (BioSource International). Assays were performed according to directions with cytokine quantity determined by comparison to standard curves developed with recombinant human cytokines and expressed as pg/ml.
IFN-γ and Granzyme B Elispot Assay
Assays were performed as described by Shafer-Weaver, et al. (13). Briefly, microplates were coated overnight with anti-human IFN-γ monoclonal antibody (BD Biosciences, San Diego, CA) or anti-human GrB antibody (clone GB-10, MABTECH Inc, Sweden) as capture antibodies and blocked with assay medium. PBMC effector cells (200,000 cells) and influenza virus-infected stimulator cells (20,000 cells) were added to triplicate wells (100 μl each). Following incubation for 20 hours at 37°C, the plates were washed and biotinylated anti-human IFN-γ or GrB detecting antibody at 2 μg/ml was then added. The plates were incubated at room temperature for two hours and washed before adding Streptavdin-HRP to each well followed by incubation for one hour at room temperature. The plates were washed again and spot forming cells were visualized using 3-Amino-9-Ethylcarbazole (AEC) substrate (BD Biosciences) for IFN-γ and 3,3′, 5,5′ tetramethylbenzidine (TMB) substrate (Moss, Inc., Pasadena, Maryland) for GrB. Spots were allowed to develop for 5-15 minutes in the dark and the reaction was then stopped by rinsing with distilled water. The membranes were air dried and spots per well were enumerated using the ImmunoSpot Imaging Analysis system (ZellNet Consulting, Inc., NY).
Data Analysis
Differences in proportion of subjects exhibiting a response were assessed in Fisher's Exact tests. Comparisons of differences between mean CTL lysis (for each E:T ratio) and cytokine concentrations were made by a two-tailed t test procedure in ANOVA using the STATVIEW Software (SAS Institute, Inc., Cary, NC). Multivariate t tests on differences between pre- and post-vaccination means of each vaccine group were also performed. For sfc analyses, a mean and standard deviation (S.D.) was calculated for PBMCs collected before vaccination; an increase in sfc greater than 2 S.D. above the baseline mean was considered significant.
Results
Reactogenicity
Reports of adverse effects from the different vaccines are shown in table 1. There were no differences in immediate pain at the vaccination site but reports of pain were significantly more common for the QS21 group at the 30 minute period postvaccination (p <.05, Fisher's Exact test). Pain was also increased in the QS21 group in the daily diary reports (p <.05, Fisher's Exact test). Myalgia reports were significantly increased in the QS21 group at the day 1 clinic visit and were also increased in the daily diary reports (p <.05 for each, Fisher's Exact test). Although not statistically significant, a number of the other adverse effects sought were increased in the QS21 vaccine group (Table 1). Adverse effects reported in the daily diaries were of one to four days' duration.
There were no severe reactions but three individuals exhibited an overall moderate reaction and one missed classes day 1 after vaccination; all three had received the QS21 containing vaccine. Other adverse effects thought attributable to the vaccine were a report of “sore arm for eight days” and presence of tenderness in the axillary region on days two to five postvaccination with a nontender lymph node palpable on this person at the day 14 visit. Both of these subjects had received the QS21 adjuvanted vaccine. There were no SAEs.
Serologic Responses
Serum HAI and neutralizing antibody responses for the two vaccine groups are shown in Table 2. The antibody response frequencies and magnitude for each antigen were high for both vaccines. There were no statistically significant differences between the two vaccines for any of the serologic responses.
Table 2.
Serum Antibody Responses to Inactivated Influenza Vaccine With or Without QS21 Adjuvant
| Hemagglutination Inhibition | Neutralization | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| GMT (log2)1 | % Rise2 | GMT (log2)1 | % Rise2 | ||||||
| Antigen | Day | Aq | QS21 | Aq | QS21 | Aq | QS21 | Aq | QS21 |
| A/Panama (H3N2) | 0 | 3.4 | 3.6 | 5.0 | 4.6 | ||||
| 14 | 7.1 | 6.1 | 10.1 | 9.4 | |||||
| 28 | 6.1 | 6.1 | 87 | 79 | 9.9 | 9.2 | 93 | 93 | |
| A/New Caledonia (H1N1) | 0 | 2.4 | 2.4 | 2.1 | 2.3 | ||||
| 14 | 5.8 | 5.5 | 6.9 | 7.3 | |||||
| 28 | 5.3 | 5.1 | 73 | 79 | 7.3 | 6.6 | 87 | 100 | |
| B/Yamanashi | 0 | 3.2 | 3.7 | 3.4 | 3.7 | ||||
| 14 | 4.9 | 6.3 | 7.2 | 8.2 | |||||
| 28 | 4.9 | 5.4 | 80 | 79 | 7.4 | 7.8 | 93 | 93 | |
Geometric mean titer
≥4-fold
CMI Responses
Mean specific cytotoxic responses (CTX) induced by ex vivo stimulation of PBMCs from vaccinees in each vaccine group are shown in Figure 1. CTX increased with increasing effector to target ratio but no significant increase over baseline was noted for the mean values for either vaccine at two weeks or four weeks or for either influenza A/Taiwan (H1N1) or influenza B/Panama when used in stimulator and target cells. Considerable variability was seen in CTX for individual subjects; some exhibited an increase over baseline (≥5%) in the 2 or 4 week bleeds and some showed a fall (data not shown).
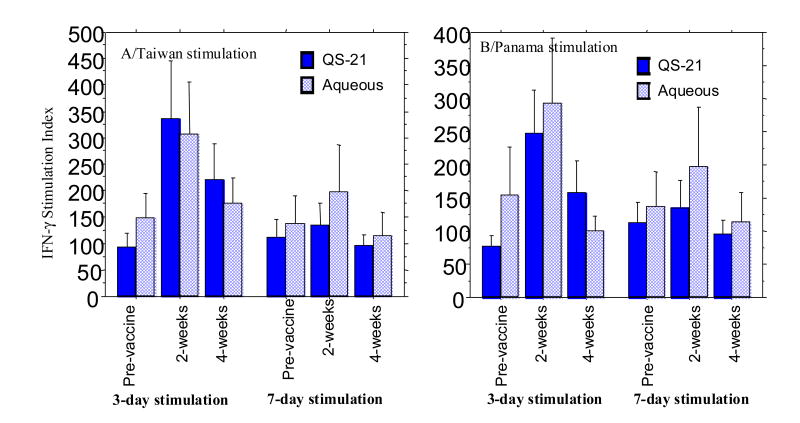
Figure 1.
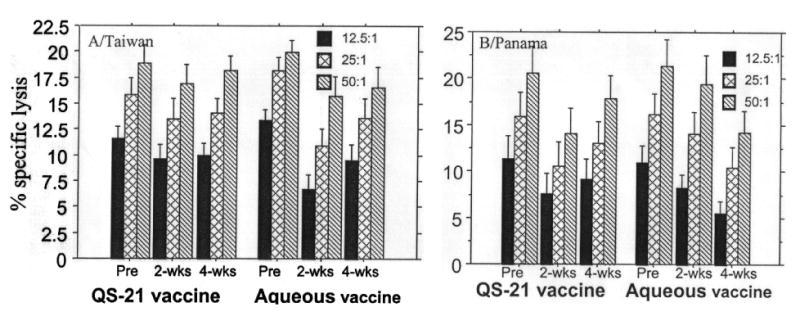
Percent specific lysis by peripheral blood mononuclear cells for infected cells at the times noted after administering inactivated influenza vaccine with (15 subjects) or without QS21 adjuvant (15 subjects). Results are means for the different effector:target cell ratios when A/Taiwan/1/86 (H1N1) or B/Panama/45/90 infected cells were used as both stimulator and target cells.
Amounts of INFγ in supernates from the CTX tests also varied considerably among the different vaccinees; however, most exhibited an increase over baseline at three days but not after seven days of in vitro stimulation. Baseline values varied between 350 and 55000 pg/ml. Because of baseline variability, the IFN-γ data were expressed as a ratio of IFN-γ in stimulated to unstimulated PBMC supernates (stimulation index). The stimulation index (SI), was significantly higher for the two week samples after three days but not seven days of culture (P <0.01, t test for day 3 paired data) for both A/Taiwan and B virus (Figure 2). However, the three day stimulation samples were not different for the two vaccines. There were no significant differences for the four week samples over baseline, either for the three or seven day stimulations. Similar analyses of IL-4 and TNFα did not show a significant change over baseline for either vaccine at any of the times (data not shown). For all comparisons, there were no significant differences between the two vaccines.
Figure 2.
Interferon gamma (IFN-γ) in supernates from the cytotoxicity assays after 3 and 7 days of stimulation with influenza A/Taiwan/86 (H1N1) or B/Panama/45/90 infected cells at the mean times shown. Because of variability in baseline values, results are shown as the mean ratio of IFN-γ in supernates from stimulated to that in unstimulated cells (stimulation index) for subjects given influenza vaccine with or without QS21 adjuvant.
To further evaluate CMI responses after vaccination, the remaining available PBMCs were used for 18 hour in vitro stimulations with autologous PBMCs infected with each of the vaccine strains followed by testing for IFN-γ and Granzyme B (Gr B) spot forming cells (sfc). Sufficient PBMCs for this testing were available from only six subjects given the QS21 containing vaccine and three given standard aqueous vaccine. Mean baseline Gr B sfc varied between 0 and 78 per 200,000 cells and baseline IFN-γ sfc varied between 0 and 217 per 200,000 cells. An increase in sfc for both Gr B and IFN-γ occurred at either two or four weeks or both after vaccination for at least two of the three antigens in each of the subjects. The increase exceeded 2 S.D. above the baseline mean in all but three instances (Table 3). Two subjects given QS21 vaccine did not exceed 2 S.D. after the influenza B stimulation (0 and 1 sfc) and one of these subjects also failed to show an increase for IFN-γ sfc after influenza A/Panama stimulation (0 sfc). The highest sfc increases were in two subjects given the QS21 vaccine (subjects 3 and 6) but there was no suggestion of a difference between vaccines for the remainder of the subjects.
Table 3.
Increases in Granzyme B and IFN-γ ELISPOTS after Inactivated Vaccine With or Without QS21 Adjuvant1
| Granzyme B sfc | IFN-γ sfc | |||||
|---|---|---|---|---|---|---|
| Vaccine Group | H32 | H12 | B2 | H32 | H12 | B2 |
| QS Subjects | ||||||
| 1 | 31 | 56 | 48 | 74 | 19 | 39 |
| 2 | 23 | 10 | 0 | 0 | 51 | 23 |
| 3 | 147 | 153 | 97 | 125 | 116 | 60 |
| 4 | 18 | 17 | 17 | 74 | 52 | 10 |
| 5 | 7 | 15 | 1 | 58 | 76 | 112 |
| 6 | 154 | 214 | 187 | 191 | 245 | 205 |
| Aqueous Subjects | ||||||
| 1 | 5 | 25 | 24 | 62 | 75 | 55 |
| 2 | 57 | 56 | 25 | 66 | 160 | 117 |
| 3 | 20 | 27 | 23 | 53 | 44 | 41 |
Mean no. sfc greater than 2 S.D. above the baseline mean; highest value at 2 or 4 weeks after vaccination
H3 = A/Panama (H3N2), H1 = A/New Caledonia (H1N1), B = B/Yamanashi
Discussion
The present study sought evidence for an immunogenicity advantage for inactivated influenza virus vaccine containing the adjuvant QS21 over standard aqueous inactivated vaccine in healthy adults. However, no advantage for QS21 adjuvanted vaccine was detected. Reactogenicity evaluations revealed a significantly greater frequency and severity of local pain at the vaccination site and of generalized myalgias among subjects given the QS21 vaccine; also, one QS21 subject missed classes for one day. However, this increased reactogenicity for the QS21 vaccine was judged as acceptable clinically if this vaccine had induced a significantly greater immune response of potential clinical benefit.
There were no differences in serum antibody responses between the vaccines for either frequency of significant increases or magnitude of the increases in either of two different serologic tests performed (HAI and neut) at either two or four weeks after vaccination. Thus, no adjuvant effect for serum antibody responses was seen in humans despite a clear superiority of QS21 adjuvant for inducing serum antibody responses in both primed and unprimed mice (7). This suggests that the mouse may not be a useful animal for discriminating between antibody responses to different inactivated influenza vaccines in humans, at least as regards value of an adjuvant.
Soon after inactivated influenza vaccines were first prepared, increased antibody responses were described in mice when Freund's or alum adjuvant were used (14). Incomplete Freund's adjuvant was incorporated into IVV and used extensively in humans in the 1950s (8). It was reported to induce significant levels of protection against interpandemic influenza and to induce antibody responses similar to aqueous vaccines for lower doses of vaccine antigen with novel, potential pandemic antigens (15). Continued use of IFA ceased at least partly because of induction of a low frequency of sterile abscesses at the vaccination site (16).
Although some increase in serum antibody responses have been reported, more recent tests in humans of IVV with an adjuvant have not clearly shown a superior response that can be expected to provide clinical benefit greater than those induced by standard aqueous vaccines (3). This has been true for use of vaccines during the interpandemic period among highly primed persons; benefit has been clearly demonstrated when adjuvant is used with IVV containing a novel, potential pandemic virus (15,17).
Since QS21 is known as a TH1 adjuvant for which a potential advantage might be induction of CMI responses after IVV, this was sought in the present study. An increase in cytotoxic lymphocytes (CTL) responses in PBMCs after IVV has been reported but these responses have been low and transient (18,19). CTL response were sought in the present study and detected in an occasional volunteer but differences between the two vaccines for different antigen stimulations at the different test times were not seen. The CTL test involved seven days of antigenic stimulation in vitro before testing for CTLs. The present results are in agreement with previous published data (20, 21) in demonstrating only minimal increases in CTL responses following administration with inactivated influenza vaccines. This is most likely due to the insensitivity of the 51Cr-release assay as previously suggested by IFN-γ ELISPOT and peptide-tetramer assays (22). All healthy adults possess baseline levels of memory influenza virus-specific CTLs due to multiple past influenza infections. Our inability to detect measurable increases in CTL responses after vaccination is probably because of expansion of memory CTLs in both pre- and post-vaccination samples during the 7 day in vitro stimulation that masks differences. Testing of supernates from this test for IFN-γ, a surrogate for CMI responses, revealed an increase in most subjects over baseline after three days of stimulation but not after seven days. Again, some subjects exhibited an increase and some did not. To further evaluate CMI, assays employing Granzyme B, a lysozomal enzyme that mediates specific CTX, and IFN-γ sfc were developed that involved only overnight stimulation of PBMCs with antigen. For this test, the three viruses used in the vaccine were used as stimulators. Unfortunately, sufficient PBMCs remained for this test on only nine of the 30 subjects. A significant increase in both GrB and IFN-γ sfc was seen in all subjects and in 51 of 54 antigen stimulation sfc tests. So, IVV clearly induced a CMI response and it appears to have done so in essentially all healthy persons. Although a greater response was seen for GrB sfc in two of the QS21 vaccinated subjects, the numbers were small and no clear advantage for the adjuvanted vaccine was identified. Influenza virus proteins known to be present in the vaccine were HA and NP but others may also have been present; the specific antigen or antigens inducing the sfc responses is unknown.
In summary, QS21 adjuvant did not enhance either humoral or CMI responses to IVV among primed healthy adults over those induced by standard 15 μg HA aqueous vaccines. The measurements used for CMI in the present study suggest that conventional seven day in vitro stimulation tests for CTLs lack sensitivity for measuring differences in CMI between humans and between influenza vaccines. The one day sfc assay for Gr B and IFN-γ appears to provide the desired sensitivity. Application of these assays should improve our understanding of CMI responses to influenza vaccines.
Acknowledgments
The authors wish to acknowledge the assistance of the following persons:
Nanette Bond
Irene Leonard
Diane Nino
Research performed by the authors and summarized in this report was supported by Public Health Service Contracts NO1-AI-65298 and NO1-AI-30039 from the National Institute of Allergy and Infectious Diseases. Work was performed at Baylor College of Medicine, Houston, Texas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
The QS-21 adjuvant was provided by Antigenics Inc.
References
- 1.Stuart-Harris CH, Schild GC, Oxford JS. Influenza, the Viruses and the Disease. Victoria, Australia and Baltimore, Maryland: Edward Arnold; 1985. Immunization with influenza virus vaccines; pp. 180–209. [Google Scholar]
- 2.Keitel WA, Couch RB. Inactivated Influenza Vaccines. In: Potter DW, editor. Influenza. Elsevier; Amsterdam: 2002. pp. 145–177. [Google Scholar]
- 3.Couch RB, Keitel WA, Cate TR. Improvement of inactivated influenza virus vaccines. J Infect Dis. 1997;176 1:S38–44. doi: 10.1086/514173. [DOI] [PubMed] [Google Scholar]
- 4.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146(2):431–7. [PubMed] [Google Scholar]
- 5.Kensil CR, Newman MJ, Coughlin RT, Soltysik S, Bedore D, Recchia J, et al. The use of Stimulon adjuvant to boost vaccine response. Vaccine Research. 1993;2(4):273–81. [Google Scholar]
- 6.Hancock GE, Speelman DJ, Frenchick PJ, Mineo-Kuhn MM, Baggs RB, Hahn DJ. Formulation of the purified fusion protein of respiratory syncytial virus with the saponin QS-21 induces protective immune responses in Balb/c mice that are similar to those generated by experimental infection. Vaccine. 1995;13(4):391–400. doi: 10.1016/0264-410x(95)98263-a. [DOI] [PubMed] [Google Scholar]
- 7.Wyde PR, Guzman E, Gilbert BE, Couch RB. Immunogenicity and protection in mice given inactivated influenza vaccine, MPL, QS-21 or QS-7. In: Osterhaus ADME, Cox N, Hampson AW, editors. Options for the Control of Influenza IV. New York: Excerpta Medica; 2001. pp. 999–1005. [Google Scholar]
- 8.Davenport FM. Seventeen years' experience with mineral oil adjuvant influenza virus vaccines. Ann Allergy. 1968;26:288–92. [PubMed] [Google Scholar]
- 9.Dowdle WN, Kendal AP, Noble GR. Influenza viruses. In: Lennette EH, Schmidt NJ, editors. Diagnostic procedures for Viral, Rickettsial and Chlamydial Infections. 5th. Washington, DC: American Public Health Association; 1979. pp. 603–5. [Google Scholar]
- 10.Frank AL, Puck J, Hughes BJ, Cate TR. Microneutralization test for influenza A and B and parainfluenza 1 and 2 viruses that uses continuous cell lines and fresh serum enhancement. J Clin Microbiol. 1980;12:426–32. doi: 10.1128/jcm.12.3.426-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mbawuike IN, Fujihashi K, DiFabio S, Kawabata W, McGhee JR, et al. Human interleukin-12 enhances interferon-γ-producing influenza-specific memory CD8+ cytotoxic T lymphocytes. J Infect Dis. 1999;180:1477–86. doi: 10.1086/315090. [DOI] [PubMed] [Google Scholar]
- 12.Mbawuike IN, Lange AR, Couch RB. Diminished influenza A virus-specific MHC class I-restricted cytotoxic T lymphocyte activity among elderly persons. Viral Immunol. 1993;6:55–64. doi: 10.1089/vim.1993.6.55. [DOI] [PubMed] [Google Scholar]
- 13.Shafer-Weaver K, Sayers T, Strobl S, Derby E, Ulderich T, Baseler M, et al. The granzyme B ELISPOT assay: an alternative to the 51Cr-release assay for monitoring cell-mediated Cytotoxicity. J Translational Med. 2003;1:14. doi: 10.1186/1479-5876-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henle W, Henle G. Effects of adjuvants on vaccination of human beings against influenza. Proc Soc Exp Bio Med. 1945;59:179–81. [Google Scholar]
- 15.Meiklejohn G. Present and future of inactivated virus vaccines. Am Rev Respir Dis. 1963;88:372–8. doi: 10.1164/arrd.1963.88.3P2.372. [DOI] [PubMed] [Google Scholar]
- 16.Medical Research Council. Influenza Vaccine Committee. Antibody responses and clinical reactions with saline and oil adjuvant influenza virus vaccines. Br Med J. 1955;2:229. [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–43. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 18.Ennis FA, Rook AH, Qi YH, Schild GC, Riley D, et al. HLA-restricted virus-specific cytotoxic T-lymphocyte responses to live and inactivated influenza vaccines. Lancet. 1981;2:887–91. doi: 10.1016/s0140-6736(81)91389-1. [DOI] [PubMed] [Google Scholar]
- 19.Gorse GJ, Belshe RB. Enhancement of anti-influenza A virus Cytotoxicity following influenza A virus vaccination in older, chronically ill adults. J Clin Microbiol. 1990;28(11):2539–50. doi: 10.1128/jcm.28.11.2539-2550.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ennis FA, Cruz J, Jameson J, Klein M, Burt D, Thipphawong J. Augmentation of human influenza A virus-specific cytotoxic T lymphocyte memory by influenza vaccine and adjuvanted carriers (ISCOMS) Virology. 1999;259:256–261. doi: 10.1006/viro.1999.9765. [DOI] [PubMed] [Google Scholar]
- 21.Powers DC. Increased immunogenicity of inactivated influenza virus vaccine containing purified surface antigen compared with whole virus in elderly women. Clin Diagn Lab Immunol. 1994;1:16–20. doi: 10.1128/cdli.1.1.16-20.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalvani A, Brookes R, Hambleton S, Britton JW, Hill AV, McMichael AJ. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]