Abstract
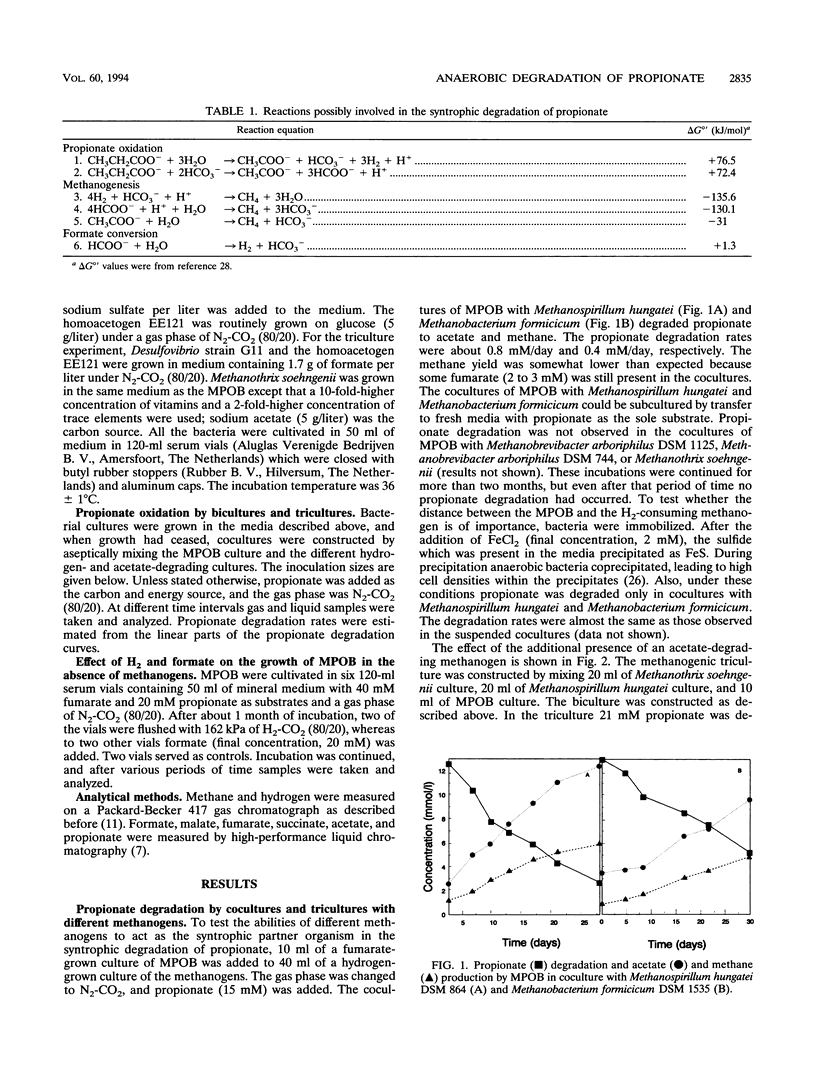
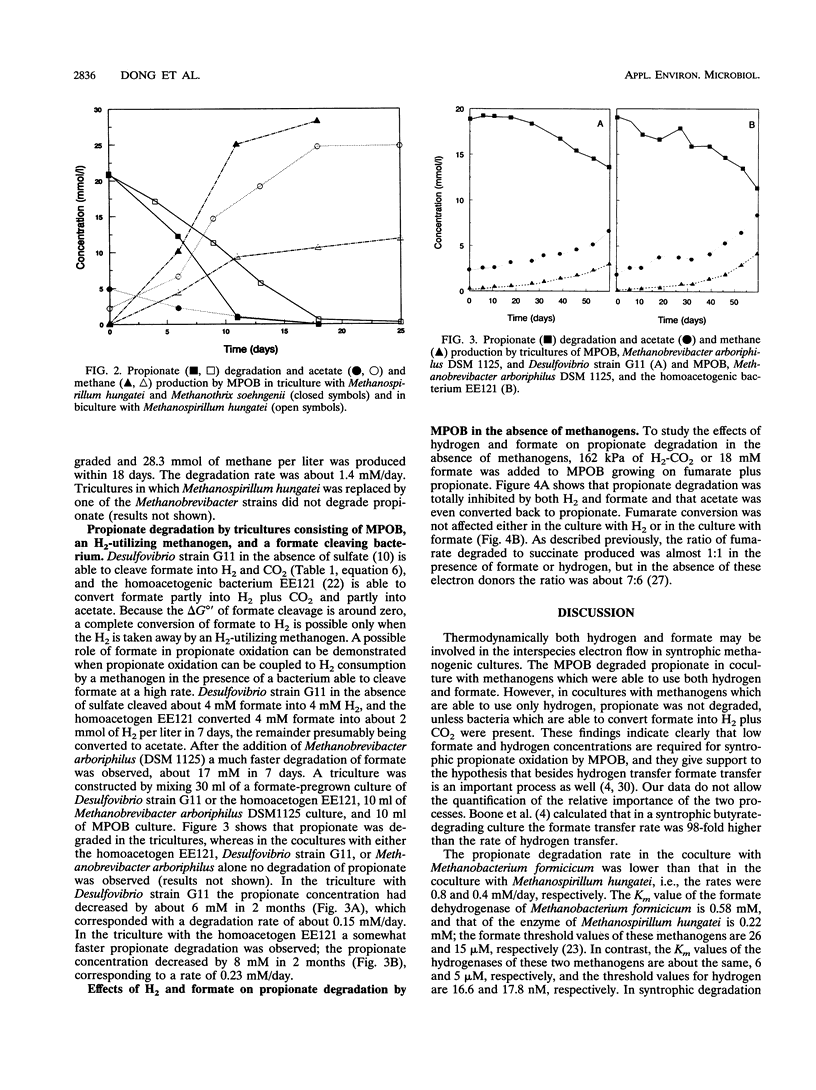
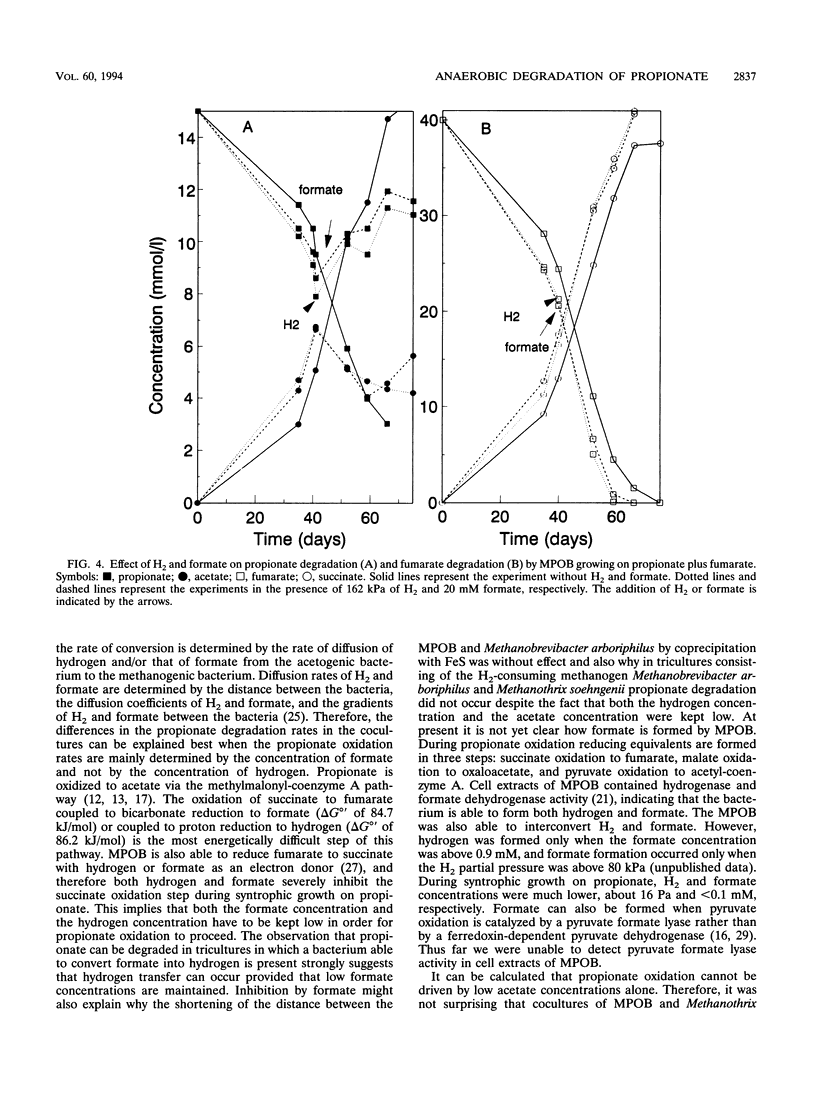
A mesophilic acetogenic bacterium (MPOB) oxidized propionate to acetate and CO2 in cocultures with the formate- and hydrogen-utilizing methanogens Methanospirillum hungatei and Methanobacterium formicicum. Propionate oxidation did not occur in cocultures with two Methanobrevibacter strains, which grew only with hydrogen. Tricultures consisting of MPOB, one of the Methanobrevibacter strains, and organisms which are able to convert formate into H2 plus CO2 (Desulfovibrio strain G11 or the homoacetogenic bacterium EE121) also degraded propionate. The MPOB, in the absence of methanogens, was able to couple propionate conversion to fumarate reduction. This propionate conversion was inhibited by hydrogen and by formate. Formate and hydrogen blocked the energetically unfavorable succinate oxidation to fumarate involved in propionate catabolism. Low formate and hydrogen concentrations are required for the syntrophic degradation of propionate by MPOB. In triculture with Methanospirillum hungatei and the aceticlastic Methanothrix soehngenii, propionate was degraded faster than in biculture with Methanospirillum hungatei, indicating that low acetate concentrations are favorable for propionate oxidation as well.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahring B. K., Westermann P. Product inhibition of butyrate metabolism by acetate and hydrogen in a thermophilic coculture. Appl Environ Microbiol. 1988 Oct;54(10):2393–2397. doi: 10.1128/aem.54.10.2393-2397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty P. S., McInerney M. J. Effects of Organic Acid Anions on the Growth and Metabolism of Syntrophomonas wolfei in Pure Culture and in Defined Consortia. Appl Environ Microbiol. 1989 Apr;55(4):977–983. doi: 10.1128/aem.55.4.977-983.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Johnson R. L., Liu Y. Diffusion of the Interspecies Electron Carriers H(2) and Formate in Methanogenic Ecosystems and Its Implications in the Measurement of K(m) for H(2) or Formate Uptake. Appl Environ Microbiol. 1989 Jul;55(7):1735–1741. doi: 10.1128/aem.55.7.1735-1741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X. Z., Schyns P. J., Stams A. J. Degradation of galactomannan by a Clostridium butyricum strain. Antonie Van Leeuwenhoek. 1991 Aug;60(2):109–114. doi: 10.1007/BF00572700. [DOI] [PubMed] [Google Scholar]
- Fukuzaki S., Nishio N., Shobayashi M., Nagai S. Inhibition of the fermentation of propionate to methane by hydrogen, acetate, and propionate. Appl Environ Microbiol. 1990 Mar;56(3):719–723. doi: 10.1128/aem.56.3.719-723.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot J. P., Brauman A. Methane Production from Formate by Syntrophic Association of Methanobacterium bryantii and Desulfovibrio vulgaris JJ. Appl Environ Microbiol. 1986 Dec;52(6):1436–1437. doi: 10.1128/aem.52.6.1436-1437.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K., Schön G. Pyruvate formate lyase in Rhodospirillum rubrum Ha adapted to anaerobic dark conditions. Arch Microbiol. 1974;99(2):109–116. doi: 10.1007/BF00696227. [DOI] [PubMed] [Google Scholar]
- Koch M., Dolfing J., Wuhrmann K., Zehnder A. J. Pathways of propionate degradation by enriched methanogenic cultures. Appl Environ Microbiol. 1983 Apr;45(4):1411–1414. doi: 10.1128/aem.45.4.1411-1414.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platen H., Schink B. Methanogenic degradation of acetone by an enrichment culture. Arch Microbiol. 1987;149(2):136–141. doi: 10.1007/BF00425079. [DOI] [PubMed] [Google Scholar]
- Schauer N. L., Brown D. P., Ferry J. G. Kinetics of Formate Metabolism in Methanobacterium formicicum and Methanospirillum hungatei. Appl Environ Microbiol. 1982 Sep;44(3):549–554. doi: 10.1128/aem.44.3.549-554.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stams A. J., Grolle K. C., Frijters C. T., Van Lier J. B. Enrichment of Thermophilic Propionate-Oxidizing Bacteria in Syntrophy with Methanobacterium thermoautotrophicum or Methanobacterium thermoformicicum. Appl Environ Microbiol. 1992 Jan;58(1):346–352. doi: 10.1128/aem.58.1.346-352.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stams A. J., Van Dijk J. B., Dijkema C., Plugge C. M. Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl Environ Microbiol. 1993 Apr;59(4):1114–1119. doi: 10.1128/aem.59.4.1114-1119.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Kirchniawy F. H., Jungermann K. A. Properties and function of the pyruvate-formate-lyase reaction in clostridiae. Eur J Biochem. 1972 May 23;27(2):282–290. doi: 10.1111/j.1432-1033.1972.tb01837.x. [DOI] [PubMed] [Google Scholar]
- Thiele Jurgen H., Zeikus J. Gregory. Control of Interspecies Electron Flow during Anaerobic Digestion: Significance of Formate Transfer versus Hydrogen Transfer during Syntrophic Methanogenesis in Flocs. Appl Environ Microbiol. 1988 Jan;54(1):20–29. doi: 10.1128/aem.54.1.20-29.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Henning D. L. Methanobacterium arbophilicum sp.nov. An obligate anaerobe isolated from wetwood of living trees. Antonie Van Leeuwenhoek. 1975;41(4):543–552. doi: 10.1007/BF02565096. [DOI] [PubMed] [Google Scholar]



