Abstract
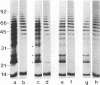
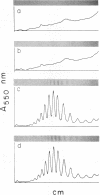
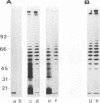
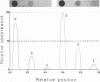
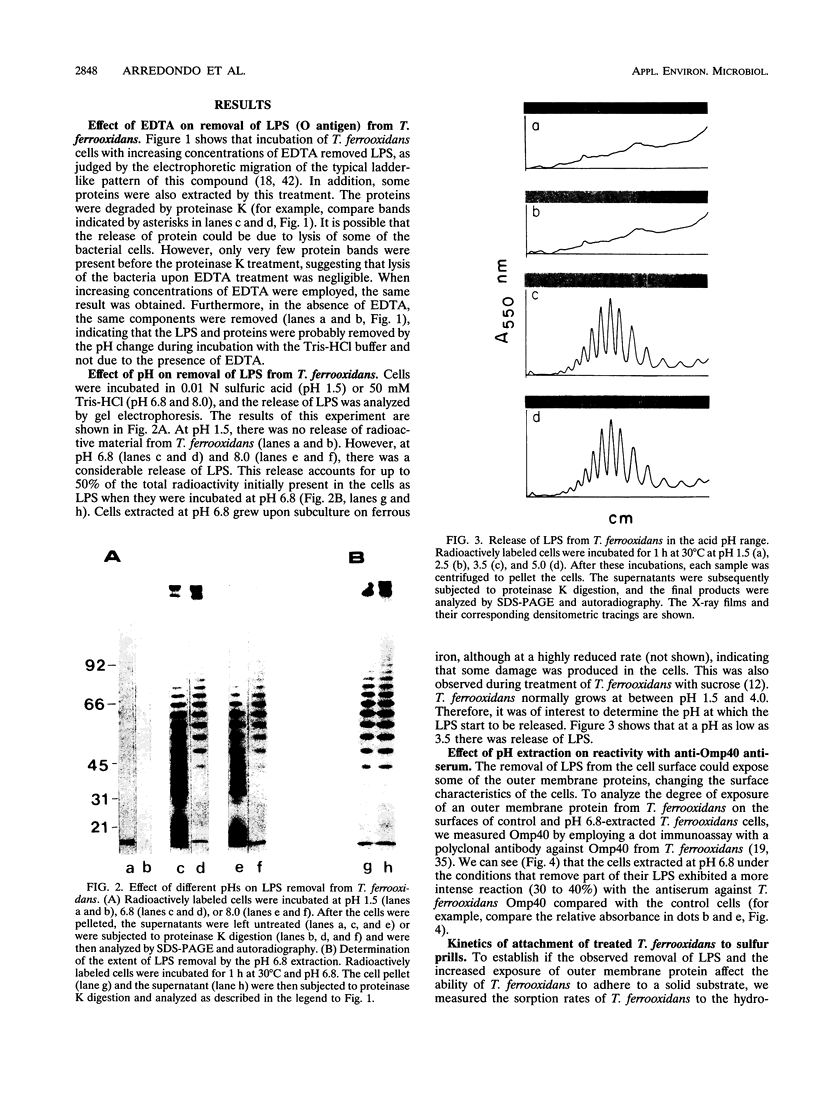
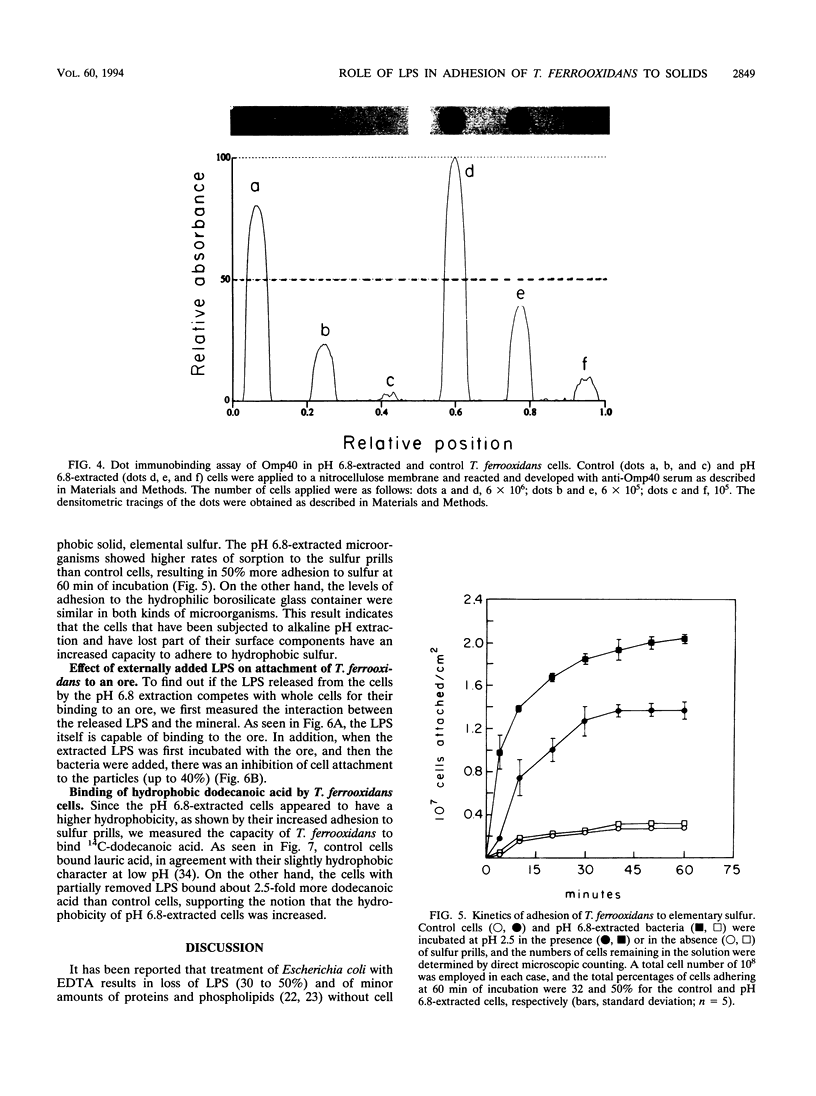
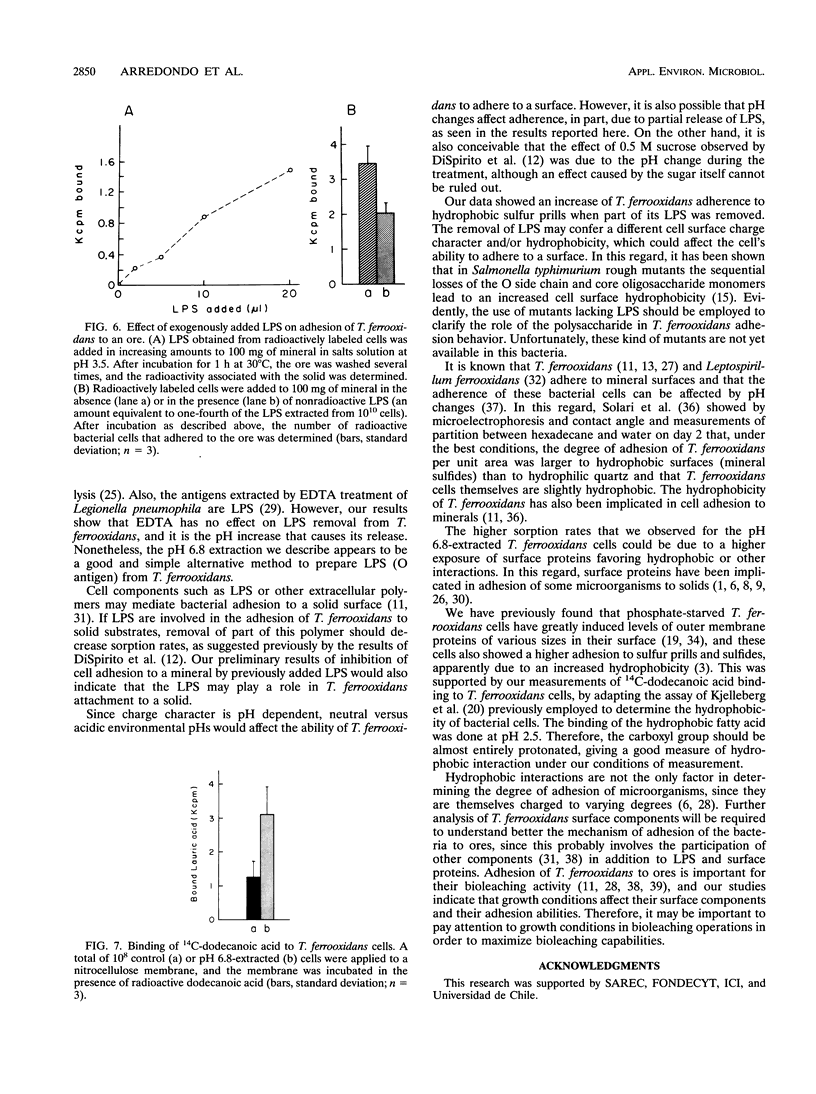
Conditions for the partial removal of lipopolysaccharide (LPS) from Thiobacillus ferrooxidans are described. Raising the pH of the solution containing the cells from pH 1.5 to pH 6.8 to 8.0 releases about 50% of the LPS without cell lysis. The release of LPS begins at pH 3.5, and it was not affected by EDTA concentration. Partial removal of LPS exposed higher amounts of a 40-kDa outer membrane protein in the bacteria, as detected by a dot immunoassay employing an antiserum against the T. ferrooxidans surface protein. This higher protein exposure and the reduced LPS content increased the hydrophobicity of the cell surface, as determined by an increased adhesion (50%) to hydrophobic sulfur prills and 14C-dodecanoic acid binding (2.5-fold) compared with control cells. In addition, adhesion of radioactively labeled microorganisms to a sulfide mineral was inhibited (40%) in the presence of previously added LPS. Our results suggest that not only LPS but also surface proteins probably play important roles in T. ferrooxidans adhesion to solid surfaces.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaro A. M., Chamorro D., Seeger M., Arredondo R., Peirano I., Jerez C. A. Effect of external pH perturbations on in vivo protein synthesis by the acidophilic bacterium Thiobacillus ferrooxidans. J Bacteriol. 1991 Jan;173(2):910–915. doi: 10.1128/jb.173.2.910-915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo Renato, Jerez Carlos A. Specific Dot-Immunobinding Assay for Detection and Enumeration of Thiobacillus ferrooxidans. Appl Environ Microbiol. 1989 Aug;55(8):2025–2029. doi: 10.1128/aem.55.8.2025-2029.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley C. L. Bacterial leaching. CRC Crit Rev Microbiol. 1978;6(3):207–26I. doi: 10.3109/10408417809090623. [DOI] [PubMed] [Google Scholar]
- Burchard R. P., Bloodgood R. A. Surface proteins of the gliding bacterium Cytophaga sp. strain U67 and its mutants defective in adhesion and motility. J Bacteriol. 1990 Jun;172(6):3379–3387. doi: 10.1128/jb.172.6.3379-3387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devasia P., Natarajan K. A., Sathyanarayana D. N., Rao G. R. Surface Chemistry of Thiobacillus ferrooxidans Relevant to Adhesion on Mineral Surfaces. Appl Environ Microbiol. 1993 Dec;59(12):4051–4055. doi: 10.1128/aem.59.12.4051-4055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Romero P. Growth of Thiobacillus ferrooxidans on Elemental Sulfur. Appl Environ Microbiol. 1987 Aug;53(8):1907–1912. doi: 10.1128/aem.53.8.1907-1912.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Costerton J. W., Macleod R. A. Separation and localization of cell wall layers of a gram-negative bacterium. J Bacteriol. 1970 Dec;104(3):1338–1353. doi: 10.1128/jb.104.3.1338-1353.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt W. E., Vestal J. R. Physical and chemical studies of Thiobacillus ferroxidans lipopolysaccharides. J Bacteriol. 1975 Aug;123(2):642–650. doi: 10.1128/jb.123.2.642-650.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerez C. A., Seeger M., Amaro A. M. Phosphate starvation affects the synthesis of outer membrane proteins in Thiobacillus ferrooxidans. FEMS Microbiol Lett. 1992 Nov 1;77(1-3):29–33. doi: 10.1016/0378-1097(92)90127-a. [DOI] [PubMed] [Google Scholar]
- Labischinski H., Barnickel G., Bradaczek H., Naumann D., Rietschel E. T., Giesbrecht P. High state of order of isolated bacterial lipopolysaccharide and its possible contribution to the permeation barrier property of the outer membrane. J Bacteriol. 1985 Apr;162(1):9–20. doi: 10.1128/jb.162.1.9-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Leive L., Shovlin V. K., Mergenhagen S. E. Physical, chemical, and immunological properties of lipopolysaccharide released from Escherichia coli by ethylenediaminetetraacetate. J Biol Chem. 1968 Dec 25;243(24):6384–6391. [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Marvin H. J., ter Beest M. B., Witholt B. Release of outer membrane fragments from wild-type Escherichia coli and from several E. coli lipopolysaccharide mutants by EDTA and heat shock treatments. J Bacteriol. 1989 Oct;171(10):5262–5267. doi: 10.1128/jb.171.10.5262-5267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura N., Kitamura K., Saiki H. Selective Adhesion of Thiobacillus ferrooxidans to Pyrite. Appl Environ Microbiol. 1993 Dec;59(12):4044–4050. doi: 10.1128/aem.59.12.4044-4050.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten S., Iyer S., Johnson W., Montgomery R. Serospecific antigens of Legionella pneumophila. J Bacteriol. 1986 Sep;167(3):893–904. doi: 10.1128/jb.167.3.893-904.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand W., Rohde K., Sobotke B., Zenneck C. Evaluation of Leptospirillum ferrooxidans for Leaching. Appl Environ Microbiol. 1992 Jan;58(1):85–92. doi: 10.1128/aem.58.1.85-92.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M., Ferreira A., Rodriguez M., Wolff D. The major Thiobacillus ferrooxidans outer membrane protein forms low conductance ion channels in planar lipid bilayers. FEBS Lett. 1992 Jan 20;296(2):169–173. doi: 10.1016/0014-5793(92)80372-n. [DOI] [PubMed] [Google Scholar]
- Southam G., Beveridge T. J. Enumeration of Thiobacilli within pH-Neutral and Acidic Mine Tailings and Their Role in the Development of Secondary Mineral Soil. Appl Environ Microbiol. 1992 Jun;58(6):1904–1912. doi: 10.1128/aem.58.6.1904-1912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam G., Beveridge T. J. Examination of Lipopolysaccharide (O-Antigen) Populations of Thiobacillus ferrooxidans from Two Mine Tailings. Appl Environ Microbiol. 1993 May;59(5):1283–1288. doi: 10.1128/aem.59.5.1283-1288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal J. R., Lundgren D. G., Milner K. C. Toxic and immunological differences among lipopolysaccharides from Thiobacillus ferrooxidans grown autotrophically and heterotrophically. Can J Microbiol. 1973 Nov;19(11):1335–1339. doi: 10.1139/m73-215. [DOI] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. Electrophoretic mobility and hydrophobicity as a measured to predict the initial steps of bacterial adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]