Abstract
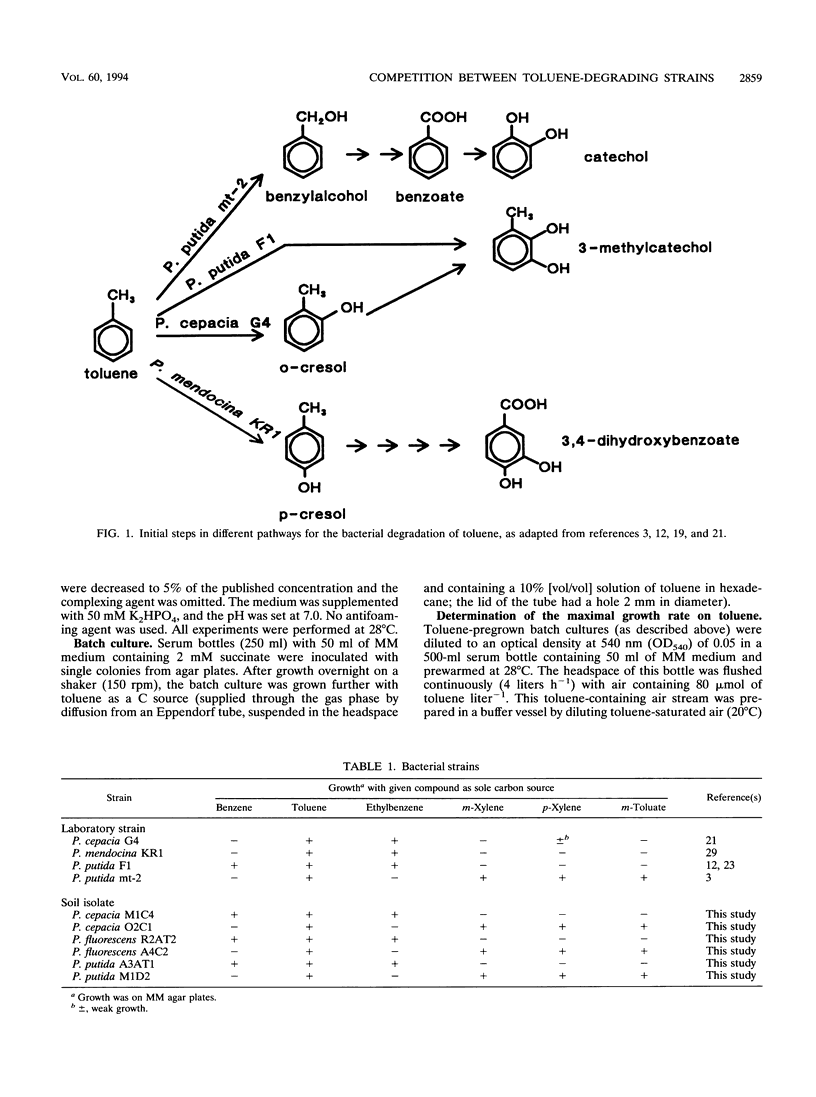
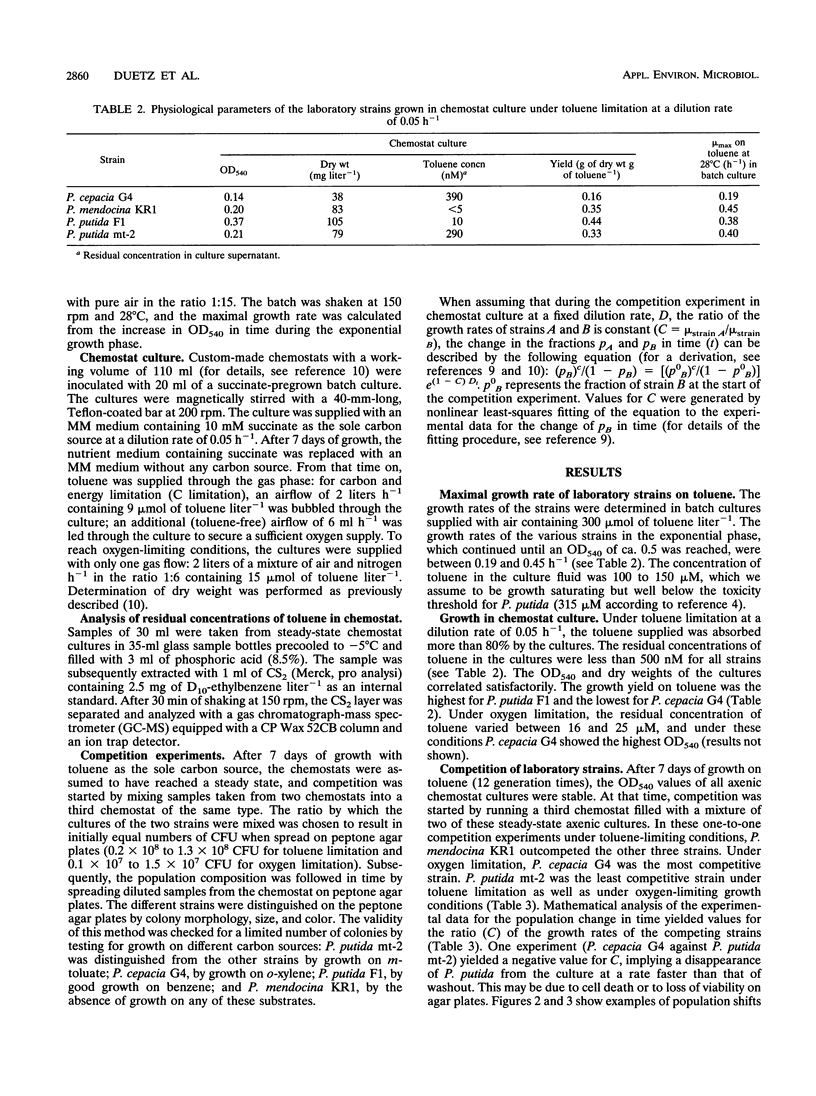
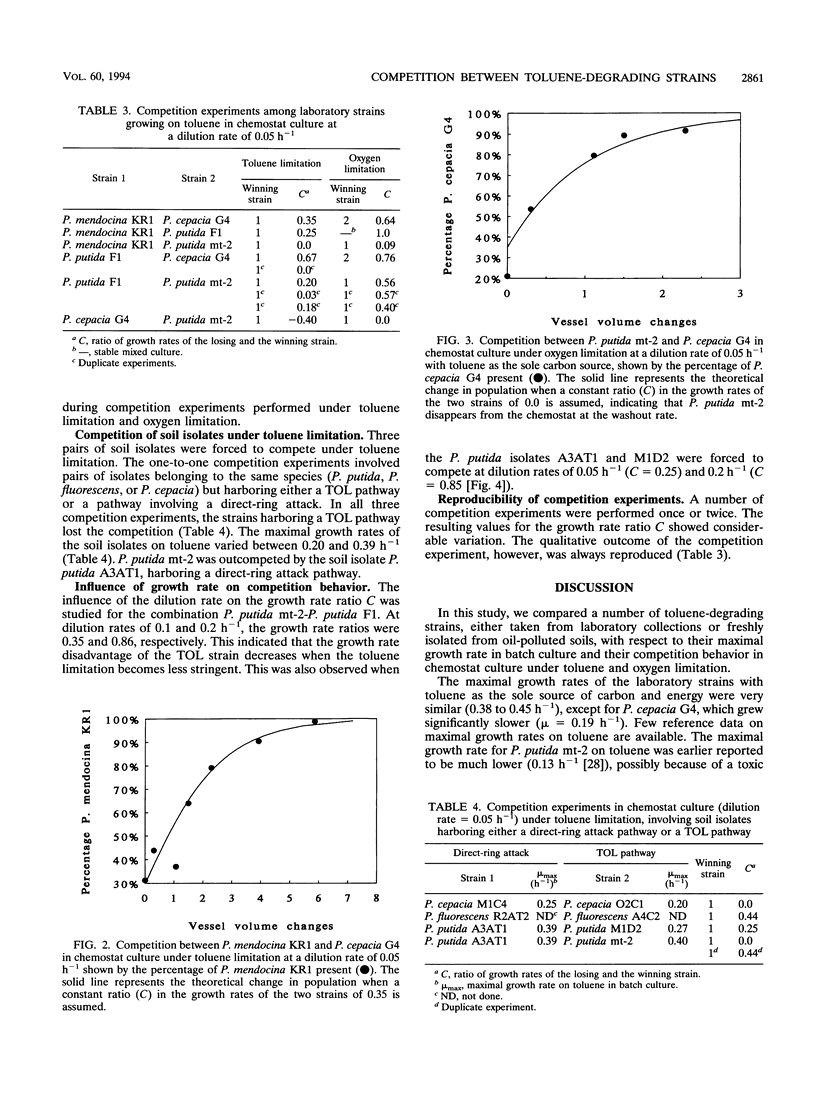
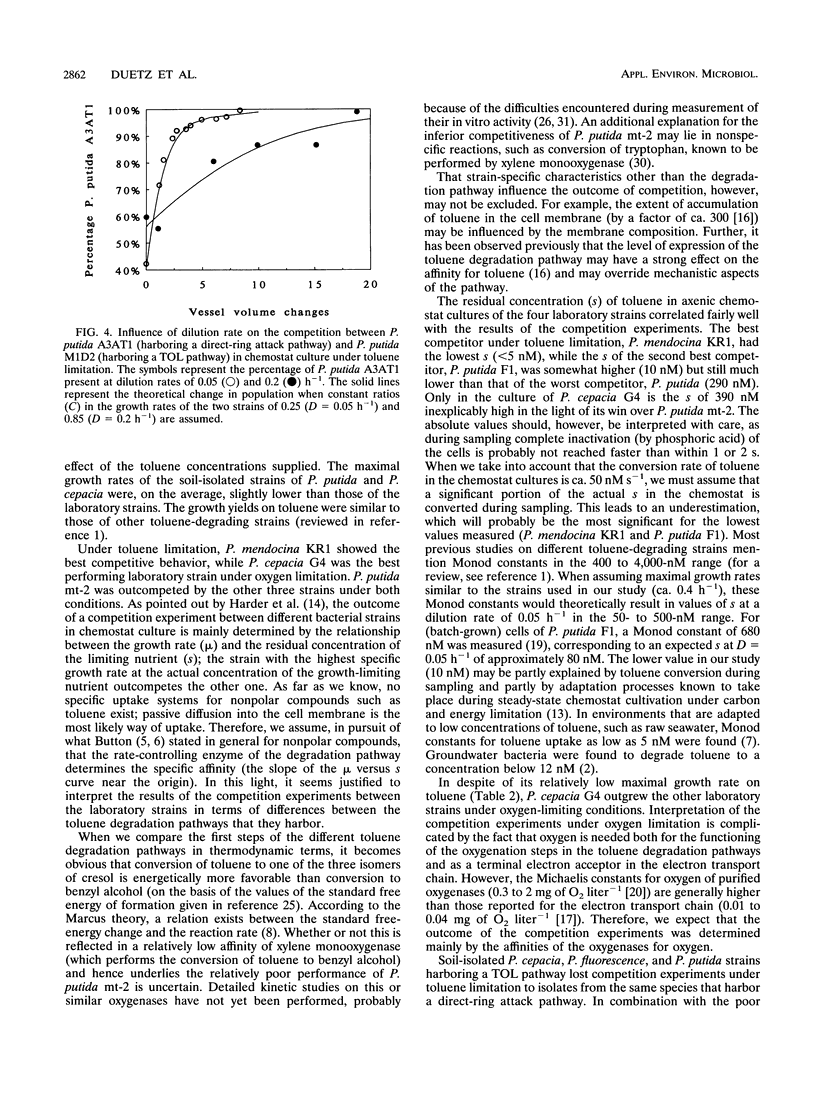
Pseudomonas putida mt-2, P. cepacia G4, P. mendocina KR1, and P. putida F1 degrade toluene through different pathways. In this study, we compared the competition behaviors of these strains in chemostat culture at a low growth rate (D = 0.05 h-1), with toluene as the sole source of carbon and energy. Either toluene or oxygen was growth limiting. Under toluene-limiting conditions, P. mendocina KR1, in which initial attack is by monooxygenation of the aromatic nucleus at the para position, outcompeted the other three strains. Under oxygen limitation, P. cepacia G4, which hydroxylates toluene in the ortho position, was the most competitive strain. P. putida mt-2, which metabolizes toluene via oxidation of the methyl group, was the least competitive strain under both growth conditions. The apparent superiority of strains carrying toluene degradation pathways that start degradation by hydroxylation of the aromatic nucleus was also found during competition experiments with pairs of strains of P. cepacia, P. fluorescence, and P. putida that were freshly isolated from contaminated soil.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez P. J., Anid P. J., Vogel T. M. Kinetics of aerobic biodegradation of benzene and toluene in sandy aquifer material. Biodegradation. 1991;2(1):43–51. doi: 10.1007/BF00122424. [DOI] [PubMed] [Google Scholar]
- Assinder S. J., Williams P. A. The TOL plasmids: determinants of the catabolism of toluene and the xylenes. Adv Microb Physiol. 1990;31:1–69. doi: 10.1016/s0065-2911(08)60119-8. [DOI] [PubMed] [Google Scholar]
- Button D. K. Kinetics of nutrient-limited transport and microbial growth. Microbiol Rev. 1985 Sep;49(3):270–297. doi: 10.1128/mr.49.3.270-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D. K. Nutrient-limited microbial growth kinetics: overview and recent advances. Antonie Van Leeuwenhoek. 1993;63(3-4):225–235. doi: 10.1007/BF00871220. [DOI] [PubMed] [Google Scholar]
- Button D. K., Robertson B. R., Craig K. S. Dissolved hydrocarbons and related microflora in a fjordal seaport: sources, sinks, concentrations, and kinetics. Appl Environ Microbiol. 1981 Oct;42(4):708–719. doi: 10.1128/aem.42.4.708-719.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duetz W. A., Winson M. K., van Andel J. G., Williams P. A. Mathematical analysis of catabolic function loss in a population of Pseudomonas putida mt-2 during non-limited growth on benzoate. J Gen Microbiol. 1991 Jun;137(6):1363–1368. doi: 10.1099/00221287-137-6-1363. [DOI] [PubMed] [Google Scholar]
- Duetz W. A., van Andel J. G. Stability of TOL plasmid pWW0 in Pseudomonas putida mt-2 under non-selective conditions in continuous culture. J Gen Microbiol. 1991 Jun;137(6):1369–1374. doi: 10.1099/00221287-137-6-1369. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Hensley M., Yoshioka H., Mabry T. J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Harder W., Kuenen J. G. A review. Microbial selection in continuous culture. J Appl Bacteriol. 1977 Aug;43(1):1–24. doi: 10.1111/j.1365-2672.1977.tb00717.x. [DOI] [PubMed] [Google Scholar]
- LONGMUIR I. S. Respiration rate of bacteria as a function of oxygen concentration. Biochem J. 1954 May;57(1):81–87. doi: 10.1042/bj0570081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law A. T., Button D. K. Modulation of affinity of a marine pseudomonad for toluene and benzene by hydrocarbon exposure. Appl Environ Microbiol. 1986 Mar;51(3):469–476. doi: 10.1128/aem.51.3.469-476.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. R., Button D. K. Toluene induction and uptake kinetics and their inclusion in the specific-affinity relationship for describing rates of hydrocarbon metabolism. Appl Environ Microbiol. 1987 Sep;53(9):2193–2205. doi: 10.1128/aem.53.9.2193-2205.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaler T. A., Klecka G. M. Effects of dissolved oxygen concentration on biodegradation of 2,4-dichlorophenoxyacetic acid. Appl Environ Microbiol. 1986 May;51(5):950–955. doi: 10.1128/aem.51.5.950-955.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields M. S., Montgomery S. O., Chapman P. J., Cuskey S. M., Pritchard P. H. Novel pathway of toluene catabolism in the trichloroethylene-degrading bacterium g4. Appl Environ Microbiol. 1989 Jun;55(6):1624–1629. doi: 10.1128/aem.55.6.1624-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R. The biodegradation of aromatic hydrocarbons by bacteria. Biodegradation. 1990;1(2-3):191–206. doi: 10.1007/BF00058836. [DOI] [PubMed] [Google Scholar]
- Spain J. C., Gibson D. T. Oxidation of substituted phenols by Pseudomonas putida F1 and Pseudomonas sp. strain JS6. Appl Environ Microbiol. 1988 Jun;54(6):1399–1404. doi: 10.1128/aem.54.6.1399-1404.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Hayakawa T., Shaw J. P., Rekik M., Harayama S. Primary structure of xylene monooxygenase: similarities to and differences from the alkane hydroxylation system. J Bacteriol. 1991 Mar;173(5):1690–1695. doi: 10.1128/jb.173.5.1690-1695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whited G. M., Gibson D. T. Separation and partial characterization of the enzymes of the toluene-4-monooxygenase catabolic pathway in Pseudomonas mendocina KR1. J Bacteriol. 1991 May;173(9):3017–3020. doi: 10.1128/jb.173.9.3017-3020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh W. K., Gibson D. T., Liu T. N. Toluene dioxygenase: a multicomponent enzyme system. Biochem Biophys Res Commun. 1977 Sep 9;78(1):401–410. doi: 10.1016/0006-291x(77)91268-2. [DOI] [PubMed] [Google Scholar]



