Abstract
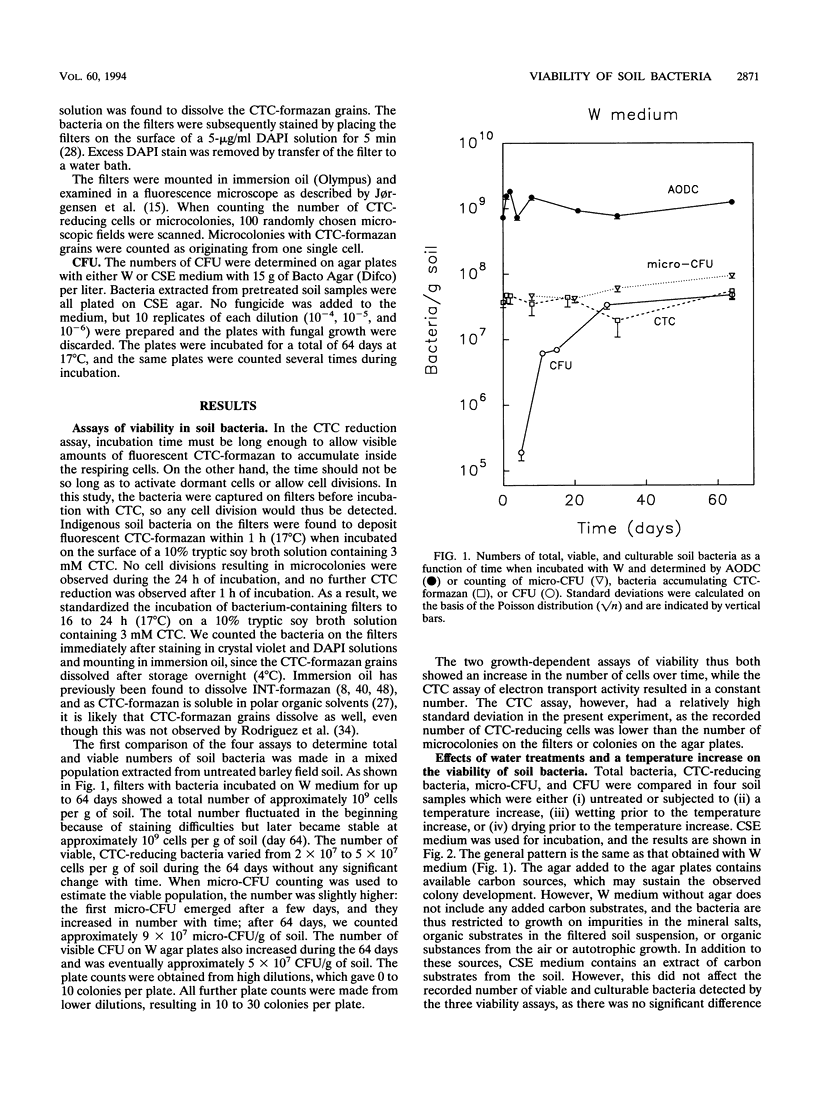
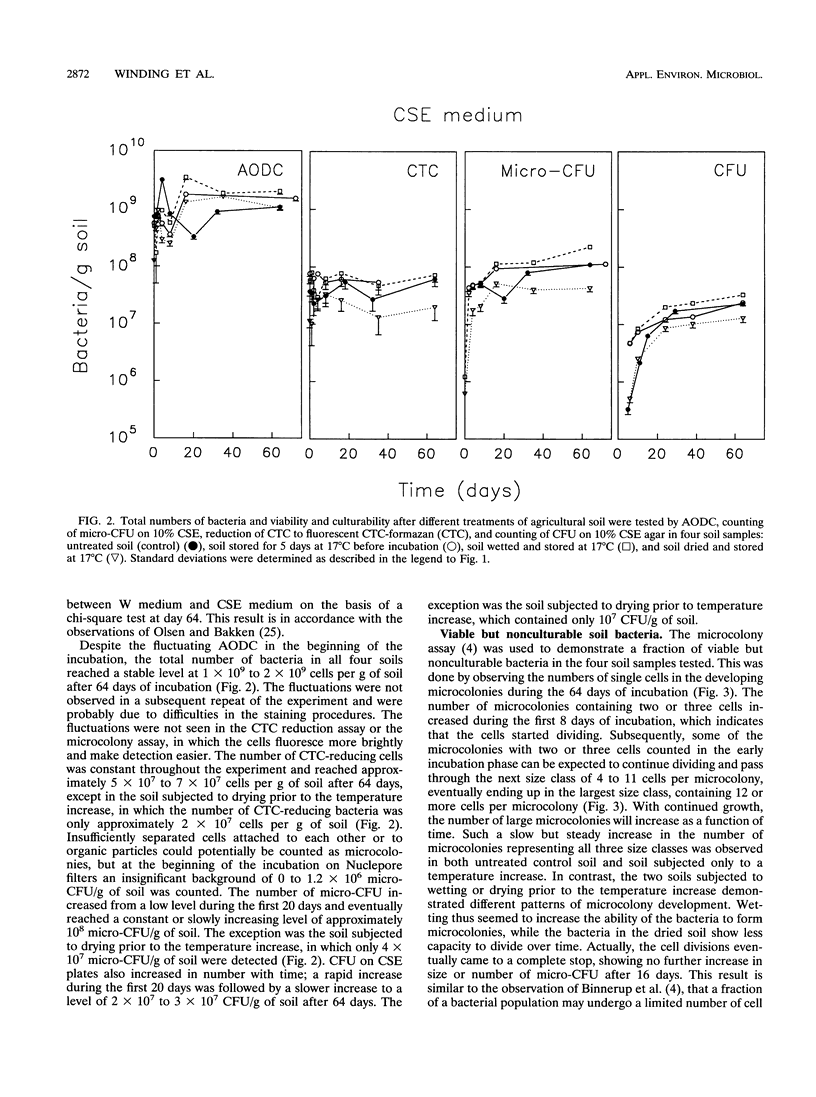
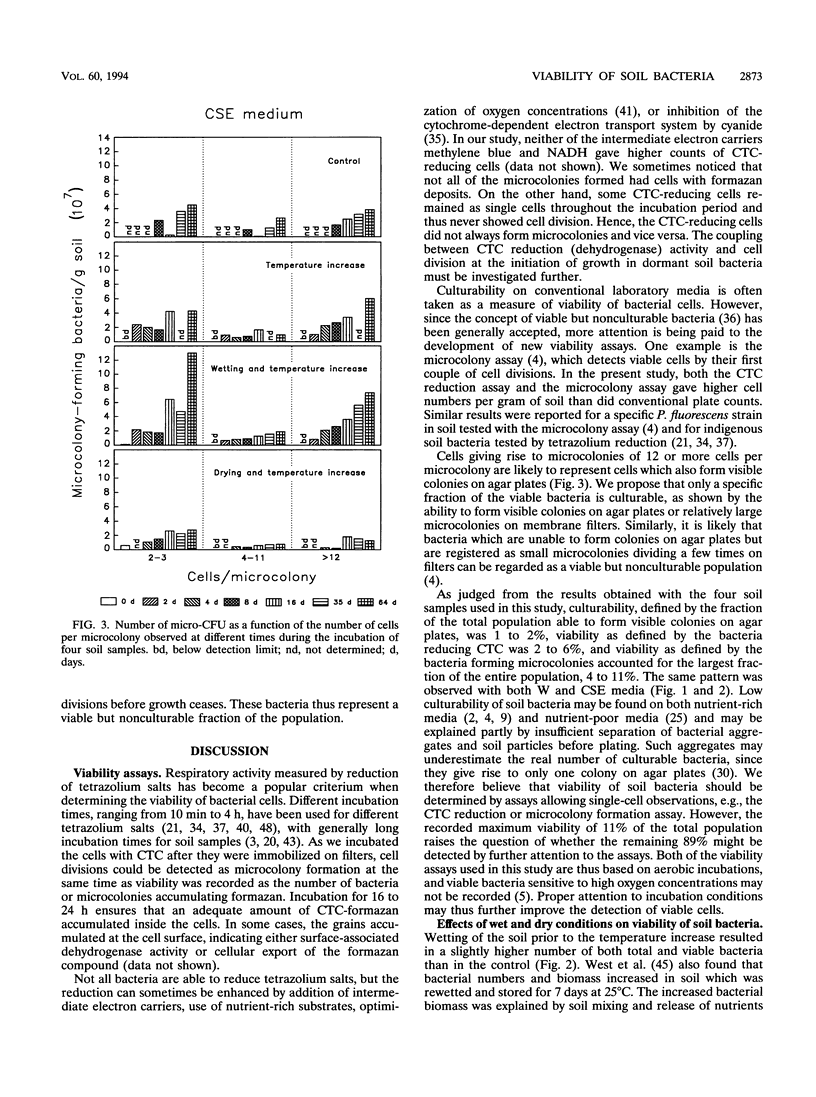
The bacterial population in barley field soil was estimated by determining the numbers of (i) cells reducing the artificial electron acceptor 5-cyano-2,3-ditolyl tetrazolium chloride (CTC) to CTC-formazan (respiratory activity), (ii) cells dividing a limited number of times (microcolony formation) on nutrient-poor media, (iii) cells dividing many times (colony formation) on nutrient-poor agar media, and (iv) cells stained with acridine orange (total counts). The CTC reduction assay was used for the first time for populations of indigenous soil bacteria and was further developed for use in this environment. The number of viable cells was highest when estimated by the number of microcolonies developing during 2 months of incubation on filters placed on the surface of nutrient-poor media. The number of bacteria reducing CTC to formazan was slightly lower than the number of bacteria forming microcolonies. Traditional plate counts of CFU (culturable cells) yielded the lowest estimate of viable cell numbers. The microcolony assay gave an estimate of both (i) cells forming true microcolonies (in which growth ceases after a few cell divisions) representing viable but nonculturable cells and (ii) cells forming larger microcolonies (in which growth continues) representing viable, culturable cells. The microcolony assay, allowing single-cell observations, thus seemed to be best suited for estimation of viable cell numbers in soil. The effect on viable and culturable cell numbers of a temperature increase from 4 to 17°C for 5 days was investigated in combination with drying or wetting of the soil. Drying or wetting prior to the temperature increase, rather than the temperature increase per se, affected both the viable and culturable numbers of bacteria; both numbers were reduced in predried soil, while they increased slightly in the prewetted soil.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitton G., Dutton R. J., Foran J. A. New rapid technique for counting microorganisms directly on membrane filters. Stain Technol. 1983 Nov;58(6):343–346. doi: 10.3109/10520298309066810. [DOI] [PubMed] [Google Scholar]
- Båth E., Frostegård A., Fritze H. Soil Bacterial Biomass, Activity, Phospholipid Fatty Acid Pattern, and pH Tolerance in an Area Polluted with Alkaline Dust Deposition. Appl Environ Microbiol. 1992 Dec;58(12):4026–4031. doi: 10.1128/aem.58.12.4026-4031.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Hattori R. The physical environment in soil microbiology: an attempt to extend principles of microbiology to soil microoganisms. CRC Crit Rev Microbiol. 1976 May;4(4):423–461. doi: 10.3109/10408417609102305. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANNASCH H. W. Studies on planktonic bacteria by means of a direct membrane filter method. J Gen Microbiol. 1958 Jun;18(3):609–620. doi: 10.1099/00221287-18-3-609. [DOI] [PubMed] [Google Scholar]
- King L. K., Parker B. C. A simple, rapid method for enumerating total viable and metabolically active bacteria in groundwater. Appl Environ Microbiol. 1988 Jun;54(6):1630–1631. doi: 10.1128/aem.54.6.1630-1631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Maki J. S., Remsen C. C. Comparison of two direct-count methods for determining metabolizing bacteria in freshwater. Appl Environ Microbiol. 1981 May;41(5):1132–1138. doi: 10.1128/aem.41.5.1132-1138.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell S. Y. Modification of the gelatin-matrix method for enumeration of respiring bacterial cells for use with salt-marsh water samples. Appl Environ Microbiol. 1984 Apr;47(4):873–875. doi: 10.1128/aem.47.4.873-875.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POSTGATE J. R., CRUMPTON J. E., HUNTER J. R. The measurement of bacterial viabilities by slide culture. J Gen Microbiol. 1961 Jan;24:15–24. doi: 10.1099/00221287-24-1-15. [DOI] [PubMed] [Google Scholar]
- Raju M. R., Carpenter S. G., Tokita N., Howard J., Lyman J. T. Biological response across a ridge filter carbon ion Bragg peak. Int J Radiat Oncol Biol Phys. 1983 Jan;9(1):67–70. doi: 10.1016/0360-3016(83)90211-0. [DOI] [PubMed] [Google Scholar]
- Roberson E. B., Firestone M. K. Relationship between Desiccation and Exopolysaccharide Production in a Soil Pseudomonas sp. Appl Environ Microbiol. 1992 Apr;58(4):1284–1291. doi: 10.1128/aem.58.4.1284-1291.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues U. M., Kroll R. G. Microcolony epifluorescence microscopy for selective enumeration of injured bacteria in frozen and heat-treated foods. Appl Environ Microbiol. 1989 Apr;55(4):778–787. doi: 10.1128/aem.55.4.778-787.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues U. M., Kroll R. G. Rapid selective enumeration of bacteria in foods using a microcolony epifluorescence microscopy technique. J Appl Bacteriol. 1988 Jan;64(1):65–78. doi: 10.1111/j.1365-2672.1988.tb02430.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez G. G., Phipps D., Ishiguro K., Ridgway H. F. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992 Jun;58(6):1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roslev P., King G. M. Application of a tetrazolium salt with a water-soluble formazan as an indicator of viability in respiring bacteria. Appl Environ Microbiol. 1993 Sep;59(9):2891–2896. doi: 10.1128/aem.59.9.2891-2896.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaule G., Flemming H. C., Ridgway H. F. Use of 5-cyano-2,3-ditolyl tetrazolium chloride for quantifying planktonic and sessile respiring bacteria in drinking water. Appl Environ Microbiol. 1993 Nov;59(11):3850–3857. doi: 10.1128/aem.59.11.3850-3857.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler E. The tetrazolium-formazan system: design and histochemistry. Prog Histochem Cytochem. 1991;24(1):1–86. doi: 10.1016/s0079-6336(11)80060-4. [DOI] [PubMed] [Google Scholar]
- Tabor P. S., Neihof R. A. Improved method for determination of respiring individual microorganisms in natural waters. Appl Environ Microbiol. 1982 Jun;43(6):1249–1255. doi: 10.1128/aem.43.6.1249-1255.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom S. M., Horobin R. W., Seidler E., Barer M. R. Factors affecting the selection and use of tetrazolium salts as cytochemical indicators of microbial viability and activity. J Appl Bacteriol. 1993 Apr;74(4):433–443. doi: 10.1111/j.1365-2672.1993.tb05151.x. [DOI] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Iturriaga R., Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978 Dec;36(6):926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



