Abstract
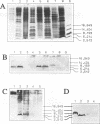
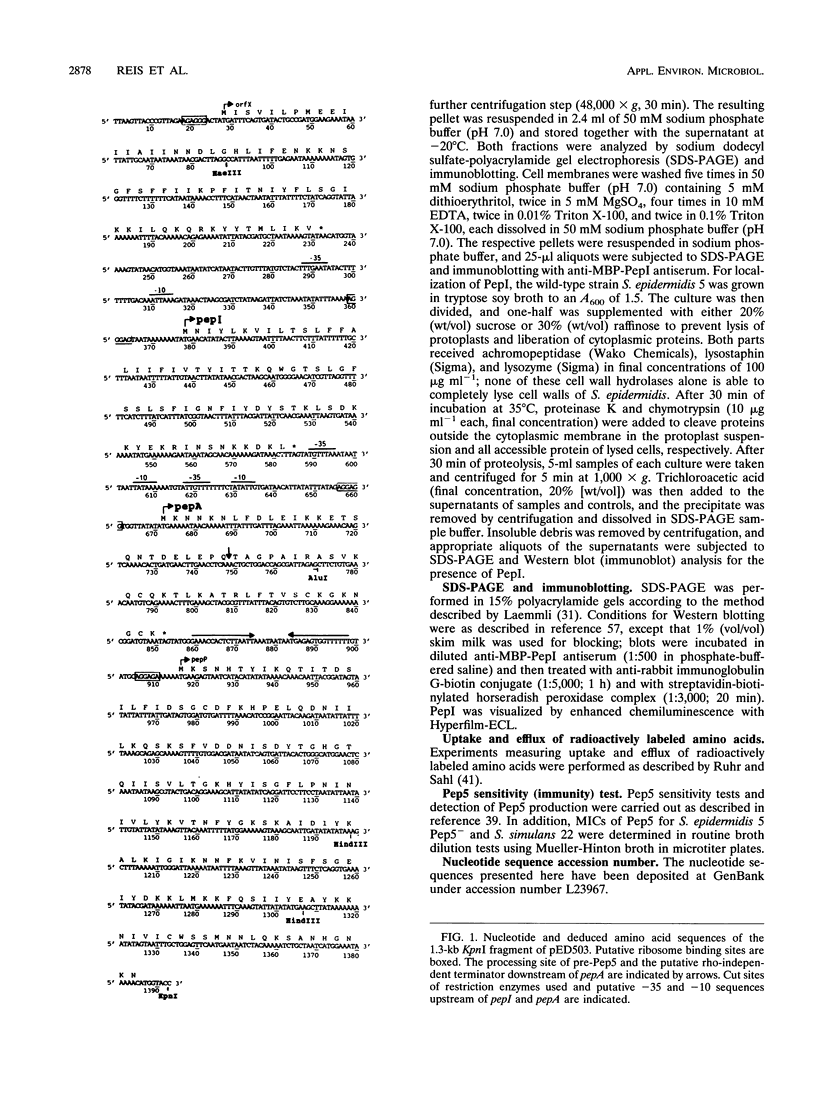
The lantibiotic Pep5 is produced by Staphylococcus epidermidis 5. Pep5 production and producer immunity are associated with the 20-kb plasmid pED503. A 1.3-kb KpnI fragment of pED503, containing the Pep5 structural gene pepA, was subcloned into the Escherichia coli-Staphylococcus shuttle vector pCU1, and the recombinant plasmid pMR2 was transferred to the Pep5- and immunity-negative mutant S. epidermidis 5 Pep5- (devoid of pED503). This clone did not produce active Pep5 but showed the same degree of insensitivity towards Pep5 as did the wild-type strain. Sequencing of the 1.3-kb KpnI-fragment and analysis of mutants demonstrated the involvement of two genes in Pep5 immunity, the structural gene pepA itself and pepI, a short open reading frame upstream of pepA. To identify the 69-amino-acid pepI gene product, we constructed an E. coli maltose-binding protein-PepI fusion clone. The immunity peptide PepI was detected in the soluble and membrane fractions of the wild-type strain and the immune mutants (harboring the plasmids pMR2 and pMR11) by immunoblotting with anti-maltose-binding protein-PepI antiserum. Strains harboring either pepI without pepA or pepI with incomplete pepA were not immune and did not produce PepI. Washing the membrane with salts and EDTA reduced the amount of PepI in this fraction, and treatment with Triton X-100 almost completely removed the peptide. Furthermore, PepI was hydrolyzed by proteases added to osmotically stabilized protoplasts. This suggests that PepI is loosely attached to the outside of the cytoplasmic membrane. Proline uptake and efflux experiments with immune and nonimmune strains also indicated that PepI may act at the membrane site.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allgaier H., Jung G., Werner R. G., Schneider U., Zähner H. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic. Eur J Biochem. 1986 Oct 1;160(1):9–22. doi: 10.1111/j.1432-1033.1986.tb09933.x. [DOI] [PubMed] [Google Scholar]
- Augustin J., Rosenstein R., Wieland B., Schneider U., Schnell N., Engelke G., Entian K. D., Götz F. Genetic analysis of epidermin biosynthetic genes and epidermin-negative mutants of Staphylococcus epidermidis. Eur J Biochem. 1992 Mar 15;204(3):1149–1154. doi: 10.1111/j.1432-1033.1992.tb16740.x. [DOI] [PubMed] [Google Scholar]
- Bierbaum G., Sahl H. G. Autolytic system of Staphylococcus simulans 22: influence of cationic peptides on activity of N-acetylmuramoyl-L-alanine amidase. J Bacteriol. 1987 Dec;169(12):5452–5458. doi: 10.1128/jb.169.12.5452-5458.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierbaum G., Sahl H. G. Induction of autolysis of staphylococci by the basic peptide antibiotics Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch Microbiol. 1985 Apr;141(3):249–254. doi: 10.1007/BF00408067. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y. J., Steen M. T., Hansen J. N. The subtilin gene of Bacillus subtilis ATCC 6633 is encoded in an operon that contains a homolog of the hemolysin B transport protein. J Bacteriol. 1992 Feb;174(4):1417–1422. doi: 10.1128/jb.174.4.1417-1422.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf F. K., Oudega B. Production and release of cloacin DF13 and related colicins. Curr Top Microbiol Immunol. 1986;125:183–205. doi: 10.1007/978-3-642-71251-7_11. [DOI] [PubMed] [Google Scholar]
- Engelke G., Gutowski-Eckel Z., Hammelmann M., Entian K. D. Biosynthesis of the lantibiotic nisin: genomic organization and membrane localization of the NisB protein. Appl Environ Microbiol. 1992 Nov;58(11):3730–3743. doi: 10.1128/aem.58.11.3730-3743.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersfeld-Dressen H., Sahl H. G., Brandis H. Plasmid involvement in production of and immunity to the staphylococcin-like peptide Pep 5. J Gen Microbiol. 1984 Nov;130(11):3029–3035. doi: 10.1099/00221287-130-11-3029. [DOI] [PubMed] [Google Scholar]
- Geli V., Baty D., Pattus F., Lazdunski C. Topology and function of the integral membrane protein conferring immunity to colicin A. Mol Microbiol. 1989 May;3(5):679–687. doi: 10.1111/j.1365-2958.1989.tb00216.x. [DOI] [PubMed] [Google Scholar]
- Gross E., Kiltz H. H., Nebelin E. Subtilin, VI. Die Struktur des Subtilins. Hoppe Seylers Z Physiol Chem. 1973 Jul;354(7):810–812. [PubMed] [Google Scholar]
- Gross E., Morell J. L. The structure of nisin. J Am Chem Soc. 1971 Sep 8;93(18):4634–4635. doi: 10.1021/ja00747a073. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hurst A., Paterson G. M. Observations on the conversion of an inactive precursor protein to the antibiotic nisin. Can J Microbiol. 1971 Nov;17(11):1379–1384. doi: 10.1139/m71-220. [DOI] [PubMed] [Google Scholar]
- Ingram L. C. Synthesis of the antibiotic nisin: formation of lanthionine and beta-methyl-lanthionine. Biochim Biophys Acta. 1969 Jun 17;184(1):216–219. doi: 10.1016/0304-4165(69)90121-4. [DOI] [PubMed] [Google Scholar]
- Ingram L. A ribosomal mechanism for synthesis of peptides related to nisin. Biochim Biophys Acta. 1970 Nov 12;224(1):263–265. doi: 10.1016/0005-2787(70)90642-8. [DOI] [PubMed] [Google Scholar]
- Kaletta C., Entian K. D., Kellner R., Jung G., Reis M., Sahl H. G. Pep5, a new lantibiotic: structural gene isolation and prepeptide sequence. Arch Microbiol. 1989;152(1):16–19. doi: 10.1007/BF00447005. [DOI] [PubMed] [Google Scholar]
- Kellner R., Jung G., Hörner T., Zähner H., Schnell N., Entian K. D., Götz F. Gallidermin: a new lanthionine-containing polypeptide antibiotic. Eur J Biochem. 1988 Oct 15;177(1):53–59. doi: 10.1111/j.1432-1033.1988.tb14344.x. [DOI] [PubMed] [Google Scholar]
- Klaenhammer T. R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993 Sep;12(1-3):39–85. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Klein C., Kaletta C., Schnell N., Entian K. D. Analysis of genes involved in biosynthesis of the lantibiotic subtilin. Appl Environ Microbiol. 1992 Jan;58(1):132–142. doi: 10.1128/aem.58.1.132-142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinkauf H., von Döhren H. Biosynthesis of peptide antibiotics. Annu Rev Microbiol. 1987;41:259–289. doi: 10.1146/annurev.mi.41.100187.001355. [DOI] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Kordel M., Benz R., Sahl H. G. Mode of action of the staphylococcinlike peptide Pep 5: voltage-dependent depolarization of bacterial and artificial membranes. J Bacteriol. 1988 Jan;170(1):84–88. doi: 10.1128/jb.170.1.84-88.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers O. P., Beerthuyzen M. M., Siezen R. J., De Vos W. M. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur J Biochem. 1993 Aug 15;216(1):281–291. doi: 10.1111/j.1432-1033.1993.tb18143.x. [DOI] [PubMed] [Google Scholar]
- Kupke T., Stevanović S., Sahl H. G., Götz F. Purification and characterization of EpiD, a flavoprotein involved in the biosynthesis of the lantibiotic epidermin. J Bacteriol. 1992 Aug;174(16):5354–5361. doi: 10.1128/jb.174.16.5354-5361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazdunski C. J., Baty D., Geli V., Cavard D., Morlon J., Lloubes R., Howard S. P., Knibiehler M., Chartier M., Varenne S. The membrane channel-forming colicin A: synthesis, secretion, structure, action and immunity. Biochim Biophys Acta. 1988 Oct 11;947(3):445–464. doi: 10.1016/0304-4157(88)90003-2. [DOI] [PubMed] [Google Scholar]
- Löfdahl S., Sjöström J. E., Philipson L. A vector for recombinant DNA in Staphylococcus aureus. Gene. 1978 Apr;3(2):161–172. doi: 10.1016/0378-1119(78)90059-8. [DOI] [PubMed] [Google Scholar]
- Mankovich J. A., Hsu C. H., Konisky J. DNA and amino acid sequence analysis of structural and immunity genes of colicins Ia and Ib. J Bacteriol. 1986 Oct;168(1):228–236. doi: 10.1128/jb.168.1.228-236.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Yamazaki N., Taniguchi H., Fujimura S. Production, purification, and properties of a bacteriocin from Staphylococcus aureus isolated from saliva. Infect Immun. 1983 Feb;39(2):609–614. doi: 10.1128/iai.39.2.609-614.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio C., Komura S., Kurahashi K. Peptide antibiotic subtilin is synthesized via precursor proteins. Biochem Biophys Res Commun. 1983 Oct 31;116(2):751–758. doi: 10.1016/0006-291x(83)90588-0. [DOI] [PubMed] [Google Scholar]
- Nissen-Meyer J., Håvarstein L. S., Holo H., Sletten K., Nes I. F. Association of the lactococcin A immunity factor with the cell membrane: purification and characterization of the immunity factor. J Gen Microbiol. 1993 Jul;139(7):1503–1509. doi: 10.1099/00221287-139-7-1503. [DOI] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell N., Entian K. D., Schneider U., Götz F., Zähner H., Kellner R., Jung G. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature. 1988 May 19;333(6170):276–278. doi: 10.1038/333276a0. [DOI] [PubMed] [Google Scholar]
- Schramm E., Olschläger T., Tröger W., Braun V. Sequence, expression and localization of the immunity protein for colicin B. Mol Gen Genet. 1988 Jan;211(1):176–182. doi: 10.1007/BF00338410. [DOI] [PubMed] [Google Scholar]
- Song H. Y., Cohen F. S., Cramer W. A. Membrane topography of ColE1 gene products: the hydrophobic anchor of the colicin E1 channel is a helical hairpin. J Bacteriol. 1991 May;173(9):2927–2934. doi: 10.1128/jb.173.9.2927-2934.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen M. T., Chung Y. J., Hansen J. N. Characterization of the nisin gene as part of a polycistronic operon in the chromosome of Lactococcus lactis ATCC 11454. Appl Environ Microbiol. 1991 Apr;57(4):1181–1188. doi: 10.1128/aem.57.4.1181-1188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens K. A., Sheldon B. W., Klapes N. A., Klaenhammer T. R. Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Appl Environ Microbiol. 1991 Dec;57(12):3613–3615. doi: 10.1128/aem.57.12.3613-3615.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Ikeda T., Mitsui I., Shiota T. Mode of inhibitory action of a bacteriocin produced by Streptococcus mutans C3603. Infect Immun. 1984 May;44(2):370–378. doi: 10.1128/iai.44.2.370-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weil H. P., Beck-Sickinger A. G., Metzger J., Stevanovic S., Jung G., Josten M., Sahl H. G. Biosynthesis of the lantibiotic Pep5. Isolation and characterization of a prepeptide containing dehydroamino acids. Eur J Biochem. 1990 Nov 26;194(1):217–223. doi: 10.1111/j.1432-1033.1990.tb19446.x. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Hayema B. J., Jeeninga R. E., Kok J., Venema G. Organization and nucleotide sequences of two lactococcal bacteriocin operons. Appl Environ Microbiol. 1991 Feb;57(2):492–498. doi: 10.1128/aem.57.2.492-498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Kok J., Venema G., Holo H., Nes I. F., Konings W. N., Abee T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J Bacteriol. 1991 Dec;173(24):7934–7941. doi: 10.1128/jb.173.24.7934-7941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. R., Polman J., Beerthuyzen M. M., Siezen R. J., Kuipers O. P., De Vos W. M. Characterization of the Lactococcus lactis nisin A operon genes nisP, encoding a subtilisin-like serine protease involved in precursor processing, and nisR, encoding a regulatory protein involved in nisin biosynthesis. J Bacteriol. 1993 May;175(9):2578–2588. doi: 10.1128/jb.175.9.2578-2588.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]