Abstract
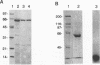
We report that 10- and 25-kDa toxin fragments adhere to CryIC prepared from Bacillus thuringiensis insecticidal crystals, block iodination, and alter membrane binding. There is no apparent affect on CryIC toxicity against Spodoptera exigua. Associated peptides remained bound to CryIC in the presence of 50 mM dithiothreitol or 6 M urea. A novel detergent-renaturation procedure was developed for the purification of B. thuringiensis CryIC toxin. Sodium dodecyl sulfate (SDS) treatment followed by gel filtration chromatography yielded a homogeneous 62-kDa CryIC toxin. After removal of SDS and renaturation, the purified CryIC toxin was fully insecticidal to S. exigua larvae. 125I-labeled CryIC bound with high affinity to brush border membrane vesicles from S. exigua larvae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum J. A., Coyle D. M., Gilbert M. P., Jany C. S., Gawron-Burke C. Novel cloning vectors for Bacillus thuringiensis. Appl Environ Microbiol. 1990 Nov;56(11):3420–3428. doi: 10.1128/aem.56.11.3420-3428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M., Plantz B. A., Swanson-Kobler J. D., Nickerson K. W. Nonenzymatic Glycosylation of Lepidopteran-Active Bacillus thuringiensis Protein Crystals. Appl Environ Microbiol. 1993 Aug;59(8):2666–2672. doi: 10.1128/aem.59.8.2666-2672.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chestukhina G. G., Tyurin S. A., Kostina L. I., Osterman A. L., Zalunin I. A., Khodova O. A., Stepanov V. M. Subdomain organization of Bacillus thuringiensis entomocidal proteins' N-terminal domains. J Protein Chem. 1990 Aug;9(4):501–507. doi: 10.1007/BF01024627. [DOI] [PubMed] [Google Scholar]
- Choma C. T., Kaplan H. Folding and unfolding of the protoxin from Bacillus thuringiensis: evidence that the toxic moiety is present in an active conformation. Biochemistry. 1990 Dec 11;29(49):10971–10977. doi: 10.1021/bi00501a015. [DOI] [PubMed] [Google Scholar]
- Choma C. T., Surewicz W. K., Carey P. R., Pozsgay M., Raynor T., Kaplan H. Unusual proteolysis of the protoxin and toxin from Bacillus thuringiensis. Structural implications. Eur J Biochem. 1990 May 20;189(3):523–527. doi: 10.1111/j.1432-1033.1990.tb15518.x. [DOI] [PubMed] [Google Scholar]
- Convents D., Houssier C., Lasters I., Lauwereys M. The Bacillus thuringiensis delta-endotoxin. Evidence for a two domain structure of the minimal toxic fragment. J Biol Chem. 1990 Jan 25;265(3):1369–1375. [PubMed] [Google Scholar]
- Ferré J., Real M. D., Van Rie J., Jansens S., Peferoen M. Resistance to the Bacillus thuringiensis bioinsecticide in a field population of Plutella xylostella is due to a change in a midgut membrane receptor. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5119–5123. doi: 10.1073/pnas.88.12.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garczynski S. F., Crim J. W., Adang M. J. Identification of putative insect brush border membrane-binding molecules specific to Bacillus thuringiensis delta-endotoxin by protein blot analysis. Appl Environ Microbiol. 1991 Oct;57(10):2816–2820. doi: 10.1128/aem.57.10.2816-2820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. S., Cowles E. A., Pietrantonio P. V. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- Hofmann C., Vanderbruggen H., Höfte H., Van Rie J., Jansens S., Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins is correlated with the presence of high-affinity binding sites in the brush border membrane of target insect midguts. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7844–7848. doi: 10.1073/pnas.85.21.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honée G., van der Salm T., Visser B. Nucleotide sequence of crystal protein gene isolated from B. thuringiensis subspecies entomocidus 60.5 coding for a toxin highly active against Spodoptera species. Nucleic Acids Res. 1988 Jul 11;16(13):6240–6240. doi: 10.1093/nar/16.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman S., Kiehne K. L., Libs J. L., Yamamoto T. Cloning of a novel cryIC-type gene from a strain of Bacillus thuringiensis subsp. galleriae. Appl Environ Microbiol. 1993 Apr;59(4):1131–1137. doi: 10.1128/aem.59.4.1131-1137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P. J., Crickmore N., Ellar D. J. The receptor for Bacillus thuringiensis CrylA(c) delta-endotoxin in the brush border membrane of the lepidopteran Manduca sexta is aminopeptidase N. Mol Microbiol. 1994 Feb;11(3):429–436. doi: 10.1111/j.1365-2958.1994.tb00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B. H., Knight P. J., Ellar D. J. N-acetyl galactosamine is part of the receptor in insect gut epithelia that recognizes an insecticidal protein from Bacillus thuringiensis. Proc Biol Sci. 1991 Jul 22;245(1312):31–35. doi: 10.1098/rspb.1991.0084. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li J. D., Carroll J., Ellar D. J. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution. Nature. 1991 Oct 31;353(6347):815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- Oddou P., Hartmann H., Radecke F., Geiser M. Immunologically unrelated Heliothis sp. and Spodoptera sp. midgut membrane-proteins bind Bacillus thuringiensis CryIA(b) delta-endotoxin. Eur J Biochem. 1993 Feb 15;212(1):145–150. doi: 10.1111/j.1432-1033.1993.tb17644.x. [DOI] [PubMed] [Google Scholar]
- Pfannenstiel M. A., Couche G. A., Muthukumar G., Nickerson K. W. Stability of the larvicidal activity of Bacillus thuringiensis subsp. israelensis: amino acid modification and denaturants. Appl Environ Microbiol. 1985 Nov;50(5):1196–1199. doi: 10.1128/aem.50.5.1196-1199.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannenstiel M. A., Cray W. C., Jr, Couche G. A., Nickerson K. W. Toxicity of protease-resistant domains from the delta-endotoxin of Bacillus thuringiensis subsp. israelensis in Culex quinquefasciatus and Aedes aegypti bioassays. Appl Environ Microbiol. 1990 Jan;56(1):162–166. doi: 10.1128/aem.56.1.162-166.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis V., Ellar D. J. Identification and partial purification of a Bacillus thuringiensis CryIC delta-endotoxin binding protein from Spodoptera littoralis gut membranes. FEBS Lett. 1993 Feb 1;316(3):264–268. doi: 10.1016/0014-5793(93)81305-j. [DOI] [PubMed] [Google Scholar]
- Sanchis V., Lereclus D., Menou G., Chaufaux J., Guo S., Lecadet M. M. Nucleotide sequence and analysis of the N-terminal coding region of the Spodoptera-active delta-endotoxin gene of Bacillus thuringiensis aizawai 7.29. Mol Microbiol. 1989 Feb;3(2):229–238. doi: 10.1111/j.1365-2958.1989.tb01812.x. [DOI] [PubMed] [Google Scholar]
- Sangadala S., Walters F. S., English L. H., Adang M. J. A mixture of Manduca sexta aminopeptidase and phosphatase enhances Bacillus thuringiensis insecticidal CryIA(c) toxin binding and 86Rb(+)-K+ efflux in vitro. J Biol Chem. 1994 Apr 1;269(13):10088–10092. [PubMed] [Google Scholar]
- Schwartz J. L., Garneau L., Savaria D., Masson L., Brousseau R., Rousseau E. Lepidopteran-specific crystal toxins from Bacillus thuringiensis form cation- and anion-selective channels in planar lipid bilayers. J Membr Biol. 1993 Feb;132(1):53–62. doi: 10.1007/BF00233051. [DOI] [PubMed] [Google Scholar]
- Tabashnik B. E., Finson N., Groeters F. R., Moar W. J., Johnson M. W., Luo K., Adang M. J. Reversal of resistance to Bacillus thuringiensis in Plutella xylostella. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4120–4124. doi: 10.1073/pnas.91.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie J., Jansens S., Höfte H., Degheele D., Van Mellaert H. Receptors on the brush border membrane of the insect midgut as determinants of the specificity of Bacillus thuringiensis delta-endotoxins. Appl Environ Microbiol. 1990 May;56(5):1378–1385. doi: 10.1128/aem.56.5.1378-1385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie J., Jansens S., Höfte H., Degheele D., Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins. Importance of specific receptors on the brush border membrane of the mid-gut of target insects. Eur J Biochem. 1989 Dec 8;186(1-2):239–247. doi: 10.1111/j.1432-1033.1989.tb15201.x. [DOI] [PubMed] [Google Scholar]
- Van Rie J., McGaughey W. H., Johnson D. E., Barnett B. D., Van Mellaert H. Mechanism of insect resistance to the microbial insecticide Bacillus thuringiensis. Science. 1990 Jan 5;247(4938):72–74. doi: 10.1126/science.2294593. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Neugebauer K. M., Lane W. S., Roth M. B. Distinct functions of SR proteins in alternative pre-mRNA splicing. Science. 1993 Apr 9;260(5105):219–222. doi: 10.1126/science.8385799. [DOI] [PubMed] [Google Scholar]