Abstract
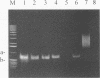
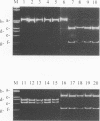
Production of the chlorosis-inducing phytotoxin coronatine in the Pseudomonas syringae pathovars atropurpurea, glycinea, maculicola, morsprunorum, and tomato has been previously reported. DNA hybridization studies previously indicated that the coronatine biosynthetic gene cluster is highly conserved among P. syringae strains which produce the toxin. In the present study, two 17-bp oligonucleotide primers derived from the coronatine biosynthetic gene cluster of P. syringae pv. glycinea PG4180 were investigated for their ability to detect coronatine-producing P. syringae strains by PCR analysis. The primer set amplified diagnostic 0.65-kb PCR products from genomic DNAs of five different coronatine-producing pathovars of P. syringae. The 0.65-kb products were not detected when PCR experiments utilized nucleic acids of nonproducers of coronatine or those of bacteria not previously investigated for coronatine production. When the 0.65-kb PCR products were digested with ClaI, PstI, and SmaI, fragments of identical size were obtained for the five different pathovars of P. syringae. A restriction fragment length polymorphism was detected in the amplified region of P. syringae pv. atropurpurea, since this pathovar lacked a conserved PvuI site which was detected in the PCR products of the other four pathovars. The 0.65-kb PCR products from six strains comprising five different pathovars of P. syringae were cloned and sequenced. The PCR products from two different P. syringae pv. glycinea strains contained identical DNA sequences, and these showed relatedness to the sequence obtained for the pathovar morsprunorum. The PCR products obtained from the pathovars maculicola and tomato were the most similar to each other, which supports the hypothesis that these two pathovars are closely related.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bej A. K., DiCesare J. L., Haff L., Atlas R. M. Detection of Escherichia coli and Shigella spp. in water by using the polymerase chain reaction and gene probes for uid. Appl Environ Microbiol. 1991 Apr;57(4):1013–1017. doi: 10.1128/aem.57.4.1013-1017.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bej A. K., Mahbubani M. H., Atlas R. M. Detection of viable Legionella pneumophila in water by polymerase chain reaction and gene probe methods. Appl Environ Microbiol. 1991 Feb;57(2):597–600. doi: 10.1128/aem.57.2.597-600.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Liyanage H., Palmer D., Ullrich M., Young S., Mitchell R. Characterization of the genes controlling the biosynthesis of the polyketide phytotoxin coronatine including conjugation between coronafacic and coronamic acid. Gene. 1993 Oct 29;133(1):31–38. doi: 10.1016/0378-1119(93)90221-n. [DOI] [PubMed] [Google Scholar]
- Bender C. L., Malvick D. K., Mitchell R. E. Plasmid-mediated production of the phytotoxin coronatine in Pseudomonas syringae pv. tomato. J Bacteriol. 1989 Feb;171(2):807–812. doi: 10.1128/jb.171.2.807-812.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Young S. A., Mitchell R. E. Conservation of Plasmid DNA Sequences in Coronatine-Producing Pathovars of Pseudomonas syringae. Appl Environ Microbiol. 1991 Apr;57(4):993–999. doi: 10.1128/aem.57.4.993-999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereswill S., Pahl A., Bellemann P., Zeller W., Geider K. Sensitive and species-specific detection of Erwinia amylovora by polymerase chain reaction analysis. Appl Environ Microbiol. 1992 Nov;58(11):3522–3526. doi: 10.1128/aem.58.11.3522-3526.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessesen M. T., Luo Q. A., Rotbart H. A., Blaser M. J., Ellison R. T., 3rd Detection of Listeria monocytogenes by using the polymerase chain reaction. Appl Environ Microbiol. 1990 Sep;56(9):2930–2932. doi: 10.1128/aem.56.9.2930-2932.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppels D. A., Moore R. A., Morris V. L. Construction and Use of a Nonradioactive DNA Hybridization Probe for Detection of Pseudomonas syringae pv. Tomato on Tomato Plants. Appl Environ Microbiol. 1990 Jun;56(6):1743–1749. doi: 10.1128/aem.56.6.1743-1749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneer H. G., Boychuk I. Species-specific detection of Listeria monocytogenes by DNA amplification. Appl Environ Microbiol. 1991 Feb;57(2):606–609. doi: 10.1128/aem.57.2.606-609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein H., Bellemann P., Walter S., Zeller W., Geider K. Identification of Erwinia amylovora, the Fireblight Pathogen, by Colony Hybridization with DNA from Plasmid pEA29. Appl Environ Microbiol. 1988 Nov;54(11):2798–2802. doi: 10.1128/aem.54.11.2798-2802.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson I. B., Mitchell R. E. Stimulation of ethylene production in bean leaf discs by the pseudomonad phytotoxin coronatine. Plant Physiol. 1985 Apr;77(4):969–973. doi: 10.1104/pp.77.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kenyon J. S., Turner J. G. The Stimulation of Ethylene Synthesis in Nicotiana tabacum Leaves by the Phytotoxin Coronatine. Plant Physiol. 1992 Sep;100(1):219–224. doi: 10.1104/pp.100.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. A., Bender C. L. Effects of Environmental and Nutritional Factors on Production of the Polyketide Phytotoxin Coronatine by Pseudomonas syringae pv. Glycinea. Appl Environ Microbiol. 1993 May;59(5):1619–1626. doi: 10.1128/aem.59.5.1619-1626.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley N. B., Gross D. C. Syringomycin production among strains of Pseudomonas syringae pv. syringae: conservation of the syrB and syrD genes and activation of phytotoxin production by plant signal molecules. Mol Plant Microbe Interact. 1994 Jan-Feb;7(1):78–90. doi: 10.1094/mpmi-7-0078. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. Polymerase chain reaction: applications in environmental microbiology. Annu Rev Microbiol. 1991;45:137–161. doi: 10.1146/annurev.mi.45.100191.001033. [DOI] [PubMed] [Google Scholar]
- Ullrich M., Bereswill S., Völksch B., Fritsche W., Geider K. Molecular characterization of field isolates of Pseudomonas syringae pv. glycinea differing in coronatine production. J Gen Microbiol. 1993 Aug;139(8):1927–1937. doi: 10.1099/00221287-139-8-1927. [DOI] [PubMed] [Google Scholar]
- Wilson I. G., Cooper J. E., Gilmour A. Detection of enterotoxigenic Staphylococcus aureus in dried skimmed milk: use of the polymerase chain reaction for amplification and detection of staphylococcal enterotoxin genes entB and entC1 and the thermonuclease gene nuc. Appl Environ Microbiol. 1991 Jun;57(6):1793–1798. doi: 10.1128/aem.57.6.1793-1798.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. A., Park S. K., Rodgers C., Mitchell R. E., Bender C. L. Physical and functional characterization of the gene cluster encoding the polyketide phytotoxin coronatine in Pseudomonas syringae pv. glycinea. J Bacteriol. 1992 Mar;174(6):1837–1843. doi: 10.1128/jb.174.6.1837-1843.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]