Abstract
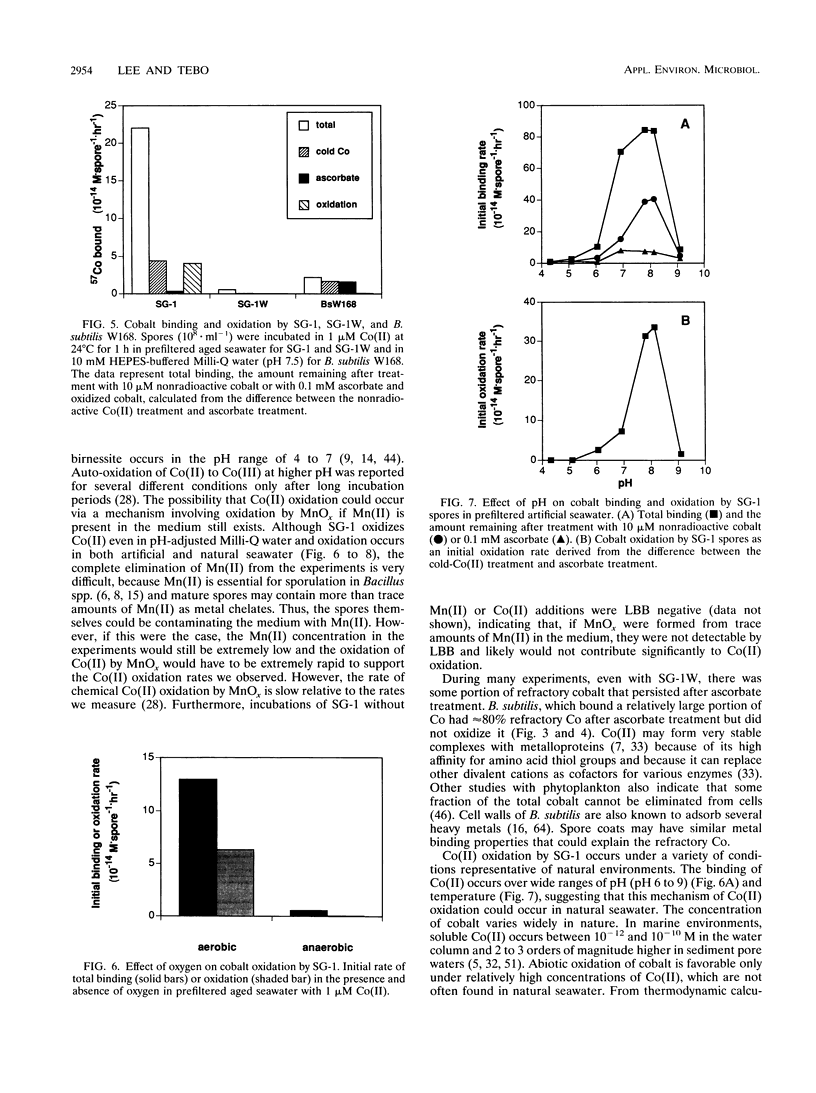
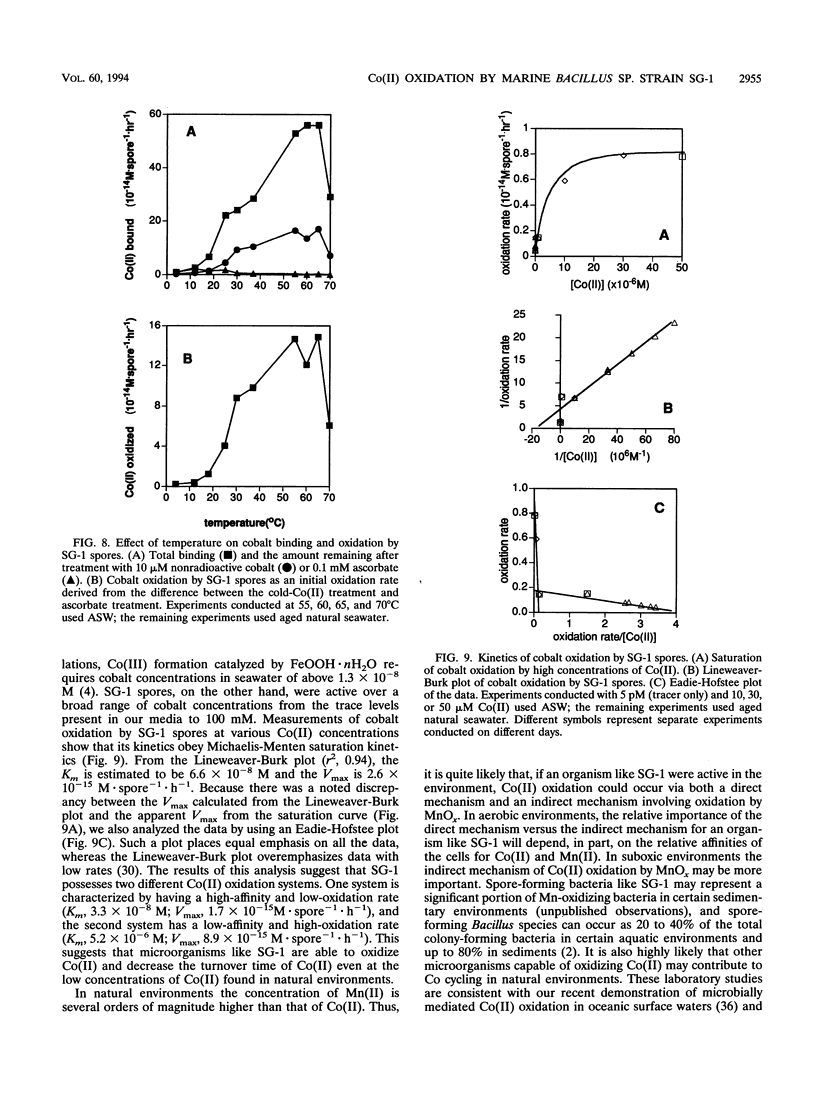
The geochemical cycling of cobalt (Co) has often been considered to be controlled by the scavenging and oxidation of Co(II) on the surface of manganese [Mn(III,IV)] oxides or manganates. Because Mn(II) oxidation in the environment is often catalyzed by bacteria, we have investigated the ability of Mn(II)-oxidizing bacteria to bind and oxidize Co(II) in the absence of Mn(II) to determine whether some Mn(II)-oxidizing bacteria also oxidize Co(II) independently of Mn oxidation. We used the marine Bacillus sp. strain SG-1, which produces mature spores that oxidize Mn(II), apparently due to a protein in their spore coats (R.A. Rosson and K. H. Nealson, J. Bacteriol. 151:1027-1034, 1982; J. P. M. de Vrind et al., Appl. Environ. Microbiol. 52:1096-1100, 1986). A method to measure Co(II) oxidation using radioactive 57Co as a tracer and treatments with nonradioactive (cold) Co(II) and ascorbate to discriminate bound Co from oxidized Co was developed. SG-1 spores were found to oxidize Co(II) over a wide range of pH, temperature, and Co(II) concentration. Leucoberbelin blue, a reagent that reacts with Mn(III,IV) oxides forming a blue color, was found to also react with Co(III) oxides and was used to verify the presence of oxidized Co in the absence of added Mn(II). Co(II) oxidation occurred optimally around pH 8 and between 55 and 65°C. SG-1 spores oxidized Co(II) at all Co(II) concentrations tested from the trace levels found in seawater to 100 mM. Co(II) oxidation was found to follow Michaelis-Menten kinetics. An Eadie-Hofstee plot of the data suggests that SG-1 spores have two oxidation systems, a high-affinity-low-rate system (Km, 3.3 × 10-8 M; Vmax, 1.7 × 10-15 M · spore-1 · h-1) and a low-affinity-high-rate system (Km, 5.2 × 10-6 M; Vmax, 8.9 × 10-15 M · spore-1 · h-1). SG-1 spores did not oxidize Co(II) in the absence of oxygen, also indicating that oxidation was not due to abiological Co(II) oxidation on the surface of preformed Mn(III,IV) oxides. These results suggest that some microorganisms may directly oxidize Co(II) and such biological activities may exert some control on the behavior of Co in nature. SG-1 spores may also have useful applications in metal removal, recovery, and immobilization processes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. F., Ghiorse W. C. Characterization of extracellular Mn2+-oxidizing activity and isolation of an Mn2+-oxidizing protein from Leptothrix discophora SS-1. J Bacteriol. 1987 Mar;169(3):1279–1285. doi: 10.1128/jb.169.3.1279-1285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARNEY J., FISHER W. P., HEGARTY C. P. Managanese as an essential element for sporulation in the genus Bacillus. J Bacteriol. 1951 Aug;62(2):145–148. doi: 10.1128/jb.62.2.145-148.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt E., Fisher S., Der C. L., Silver S. Manganese transport in Bacillus subtilis W23 during growth and sporulation. J Bacteriol. 1973 Mar;113(3):1363–1372. doi: 10.1128/jb.113.3.1363-1372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming C. A., Ferris F. G., Beveridge T. J., Bailey G. W. Remobilization of toxic heavy metals adsorbed to bacterial wall-clay composites. Appl Environ Microbiol. 1990 Oct;56(10):3191–3203. doi: 10.1128/aem.56.10.3191-3203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D. Carboxypeptidase E. Annu Rev Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Tipper D. J. Bacillus subtilis spore coats: complexity and purification of a unique polypeptide component. J Bacteriol. 1978 Sep;135(3):1091–1106. doi: 10.1128/jb.135.3.1091-1106.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Merendino K. A., Winterscheid L. C., Dillard D. H. Cystic medial necrosis with and without Marfan's syndrome. Surgical experience with 20 patients and a note about the modified bicuspidization operation. Surg Clin North Am. 1967 Dec;47(6):1403–1418. doi: 10.1016/s0039-6109(16)38390-6. [DOI] [PubMed] [Google Scholar]
- Nucho R., Baudin J. P. 60Co retention by a freshwater planktonic alga Scenedesmus obliquus. Environ Pollut. 1989;62(4):265–279. doi: 10.1016/0269-7491(89)90149-8. [DOI] [PubMed] [Google Scholar]
- Rosson R. A., Nealson K. H. Manganese binding and oxidation by spores of a marine bacillus. J Bacteriol. 1982 Aug;151(2):1027–1034. doi: 10.1128/jb.151.2.1027-1034.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui A. Nickel and copper accumplation as essential elements in 10-A manganite of deep-sea manganese nodules. Nature. 1979 May 31;279(5712):411–413. doi: 10.1038/279411a0. [DOI] [PubMed] [Google Scholar]
- Walker S. G., Flemming C. A., Ferris F. G., Beveridge T. J., Bailey G. W. Physicochemical interaction of Escherichia coli cell envelopes and Bacillus subtilis cell walls with two clays and ability of the composite to immobilize heavy metals from solution. Appl Environ Microbiol. 1989 Nov;55(11):2976–2984. doi: 10.1128/aem.55.11.2976-2984.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrind J. P., de Vrind-de Jong E. W., de Voogt J. W., Westbroek P., Boogerd F. C., Rosson R. A. Manganese oxidation by spores and spore coats of a marine bacillus species. Appl Environ Microbiol. 1986 Nov;52(5):1096–1100. doi: 10.1128/aem.52.5.1096-1100.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Waasbergen L. G., Hoch J. A., Tebo B. M. Genetic analysis of the marine manganese-oxidizing Bacillus sp. strain SG-1: protoplast transformation, Tn917 mutagenesis, and identification of chromosomal loci involved in manganese oxidation. J Bacteriol. 1993 Dec;175(23):7594–7603. doi: 10.1128/jb.175.23.7594-7603.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]