Abstract
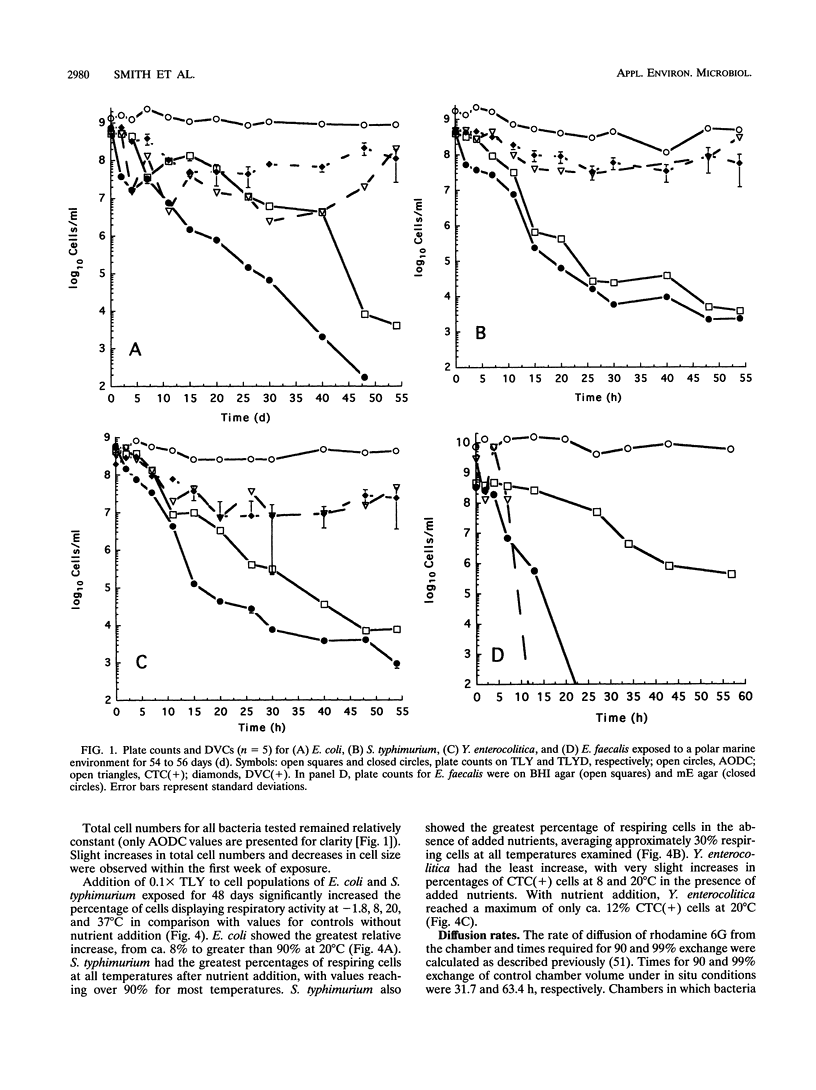
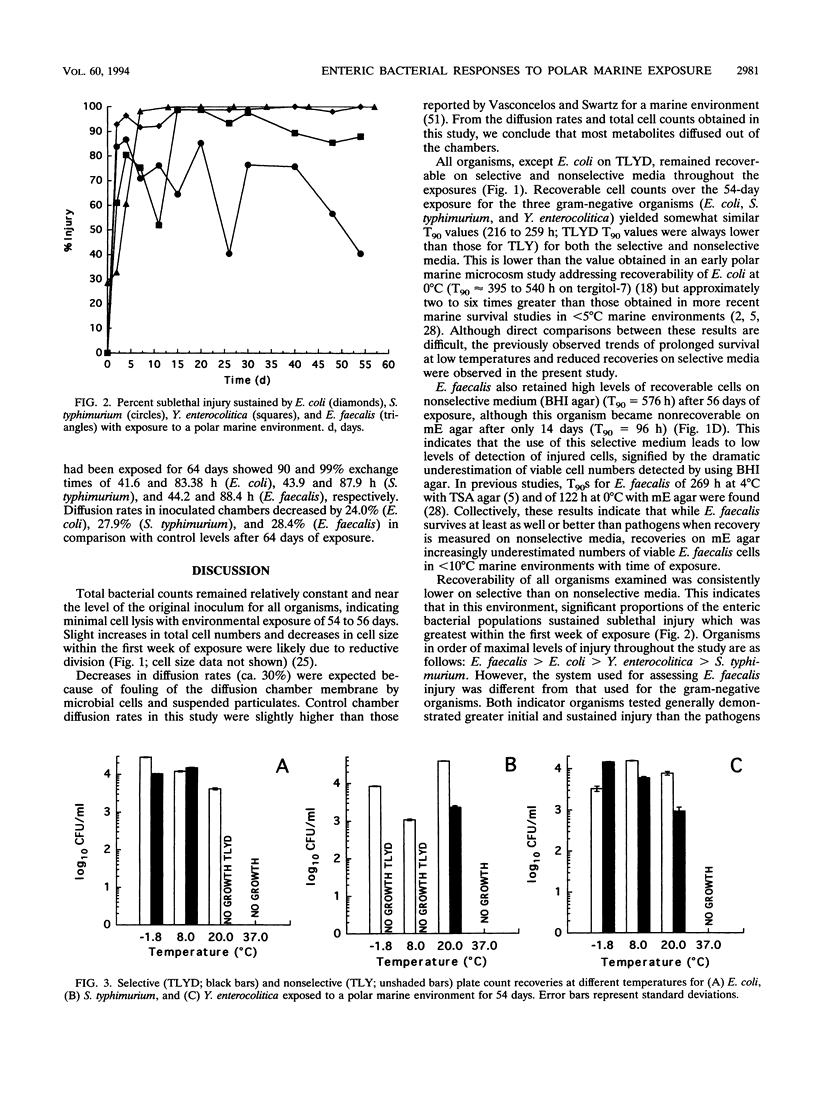
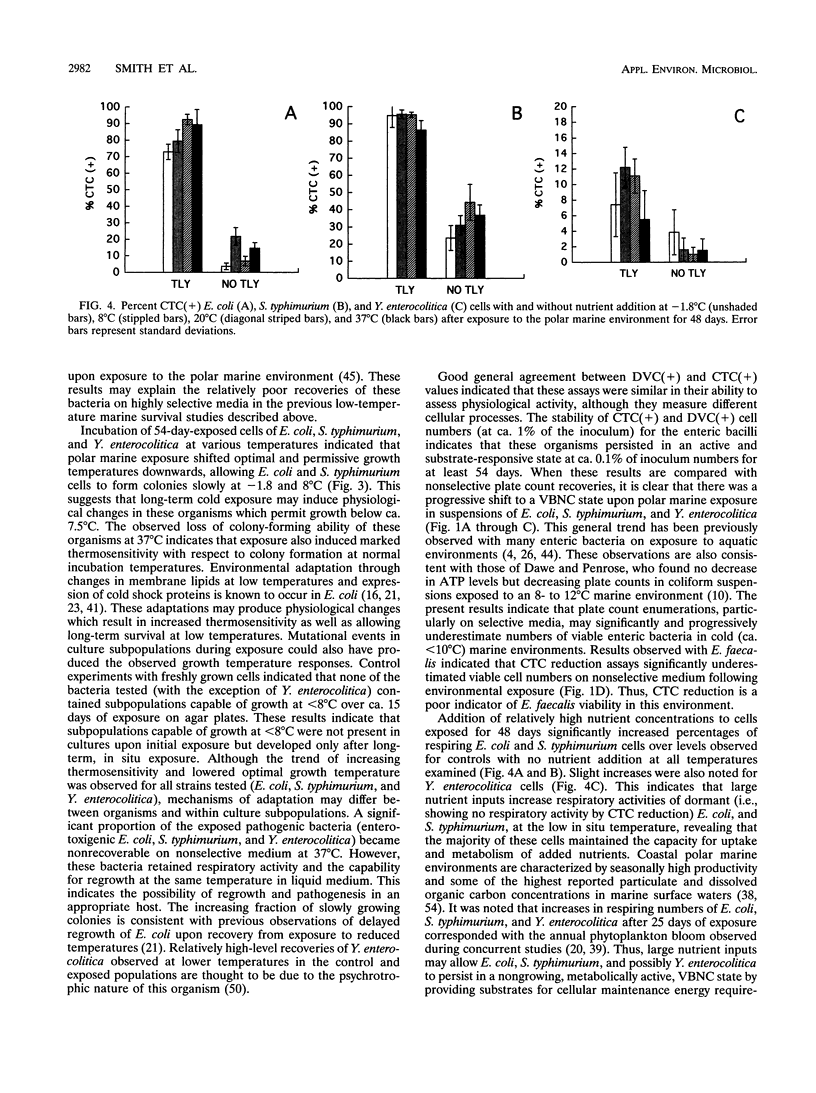
Survival, sublethal injury, and recoverability of Escherichia coli, Enterococcus faecalis, Salmonella typhimurium, and Yersinia enterocolitica were investigated by using diffusion chambers over 54 to 56 days of in situ exposure to a polar marine environment (-1.8 degrees C; salinity, 34.5 ppt) at McMurdo Station, Antarctica. Plate counts were used to assess recoverability and injury, whereas direct viable counts (DVCs) and 5-cyano-2,3-ditolyl tetrazolium chloride (CTC) reduction were utilized to determine substrate responsiveness and respiratory activity, respectively. T90 values (times for 10-fold decreases in numbers of recoverable cells) on nonselective medium were ca. 216 to 259 h for E. coli, S. typhimurium, and Y. enterocolitica and 432 h for E. faecalis. Sublethal injury was greater in populations of indicator bacteria than in pathogens. DVCs, CTC reduction, and plate counts indicated progressive increases in viable but nonculturable cells in E. coli, S. typhimurium, and Y. enterocolitica cultures throughout the 54-day exposure. Forty-eight-day exposure of E. coli, S. typhimurium, and Y. enterocolitica resulted in decreased optimal incubation temperatures for colony formation and inability to form colonies at 37 degrees C. The detection of responsive E. coli, S. typhimurium, and Y. enterocolitica by the DVC and CTC methods remained within 1% of inoculum values during 54 days of exposure, indicating some long-term persistence in the viable-but-nonculturable state. Percentages of respiring E. coli and S. typhimurium increased significantly upon addition of nutrients at all temperatures tested, indicating that nutrient availability rather than temperature limited enteric bacterial activity in this very cold environment. Large nutrient inputs to low-temperature marine environments may thus allow for the long-term persistence of enteric bacteria in a nonrecoverable state.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Rhodes M. W., Kator H. I. Seasonal variation in survival of Escherichia coli exposed in situ in membrane diffusion chambers containing filtered and nonfiltered estuarine water. Appl Environ Microbiol. 1983 Jun;45(6):1877–1883. doi: 10.1128/aem.45.6.1877-1883.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcina I., González J. M., Iriberri J., Egea L. Survival strategy of Escherichia coli and Enterococcus faecalis in illuminated fresh and marine systems. J Appl Bacteriol. 1990 Feb;68(2):189–198. doi: 10.1111/j.1365-2672.1990.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Baross J. A., Hanus F. J., Morita R. Y. Survival of human enteric and other sewage microorganisms under simulated deep-sea conditions. Appl Microbiol. 1975 Aug;30(2):309–318. doi: 10.1128/am.30.2.309-318.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeze R. J., Solomon C. J., Pope D. H. Effects of low temperature on in vivo and in vitro protein synthesis in Escherichia coli and Pseudomonas fluorescens. J Bacteriol. 1978 Jun;134(3):861–874. doi: 10.1128/jb.134.3.861-874.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLUCCI A. F., PRAMER D. Factors affecting the survival of bacteria in sea water. Appl Microbiol. 1959 Nov;7:388–392. doi: 10.1128/am.7.6.388-392.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe L. L., Penrose W. R. "Bactericidal" property of seawater: death or debilitation? Appl Environ Microbiol. 1978 May;35(5):829–833. doi: 10.1128/aem.35.5.829-833.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust M. A., Aotaky A. E., Hargadon M. T. Effect of physical parameters on the in situ survival of Escherichia coli MC-6 in an estuarine environment. Appl Microbiol. 1975 Nov;30(5):800–806. doi: 10.1128/am.30.5.800-806.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R. L., Buckley E. N., Palumbo A. V. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984 Jan;47(1):49–55. doi: 10.1128/aem.47.1.49-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier M. J., Flatau G. N., Clément R. L., Munro P. M. Sensitivity of Escherichia coli cells to seawater closely depends on their growth stage. J Appl Bacteriol. 1992 Sep;73(3):257–262. [PubMed] [Google Scholar]
- Goodrich T. D., Morita R. Y. Low temperature inhibition on binding, trasport, and incorporation of leucine, arginine, methionine, and histidine in Escherichia coli). Z Allg Mikrobiol. 1977;17(2):91–97. [PubMed] [Google Scholar]
- Gounot A. M. Bacterial life at low temperature: physiological aspects and biotechnological implications. J Appl Bacteriol. 1991 Nov;71(5):386–397. doi: 10.1111/j.1365-2672.1991.tb03806.x. [DOI] [PubMed] [Google Scholar]
- Halton J. E., Nehlsen W. R. Survival of Escherichia coli in zero-degree centigrade sea water. J Water Pollut Control Fed. 1968 May;40(5):865–868. [PubMed] [Google Scholar]
- Harker C. Bacteriological examination of the water supply on an Antarctic base. Epidemiol Infect. 1989 Feb;102(1):105–112. doi: 10.1017/s0950268800029733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. G., Cashel M., Glaser G., Neidhardt F. C. Function of a relaxed-like state following temperature downshifts in Escherichia coli. J Bacteriol. 1992 Jun;174(12):3903–3914. doi: 10.1128/jb.174.12.3903-3914.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprelyants A. S., Gottschal J. C., Kell D. B. Dormancy in non-sporulating bacteria. FEMS Microbiol Rev. 1993 Apr;10(3-4):271–285. doi: 10.1111/j.1574-6968.1993.tb05871.x. [DOI] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M., Mårdén P., Jones G. W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- Knight I. T., Shults S., Kaspar C. W., Colwell R. R. Direct detection of Salmonella spp. in estuaries by using a DNA probe. Appl Environ Microbiol. 1990 Apr;56(4):1059–1066. doi: 10.1128/aem.56.4.1059-1066.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Lessard E. J., Sieburth J. M. Survival of natural sewage populations of enteric bacteria in diffusion and batch chambers in the marine environment. Appl Environ Microbiol. 1983 Mar;45(3):950–959. doi: 10.1128/aem.45.3.950-959.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFeters G. A., Stuart D. G. Survival of coliform bacteria in natural waters: field and laboratory studies with membrane-filter chambers. Appl Microbiol. 1972 Nov;24(5):805–811. doi: 10.1128/am.24.5.805-811.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro P. M., Gauthier M. J., Breittmayer V. A., Bongiovanni J. Influence of osmoregulation processes on starvation survival of Escherichia coli in seawater. Appl Environ Microbiol. 1989 Aug;55(8):2017–2024. doi: 10.1128/aem.55.8.2017-2024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L., Oliver J. D., Kjelleberg S. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J Bacteriol. 1991 Aug;173(16):5054–5059. doi: 10.1128/jb.173.16.5054-5059.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qoronfleh M. W., Debouck C., Keller J. Identification and characterization of novel low-temperature-inducible promoters of Escherichia coli. J Bacteriol. 1992 Dec;174(24):7902–7909. doi: 10.1128/jb.174.24.7902-7909.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez G. G., Phipps D., Ishiguro K., Ridgway H. F. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992 Jun;58(6):1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Pyle B. H., McFeters G. A. Rapid enumeration of viable bacteria by image analysis. J Microbiol Methods. 1989;10:91–101. doi: 10.1016/0167-7012(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Sullivan C. W., Palmisano A. C. Sea Ice Microbial Communities: Distribution, Abundance, and Diversity of Ice Bacteria in McMurdo Sound, Antarctica, in 1980. Appl Environ Microbiol. 1984 Apr;47(4):788–795. doi: 10.1128/aem.47.4.788-795.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos G. J., Swartz R. G. Survival of bacteria in seawater using a diffusion chamber apparatus in situ. Appl Environ Microbiol. 1976 Jun;31(6):913–920. doi: 10.1128/aem.31.6.913-920.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran K., Takai T., Navarro I. M., Nakano H., Hashimoto H., Siebeling R. J. Ecology of Vibrio cholerae non-O1 and Salmonella spp. and role of zooplankton in their seasonal distribution in Fukuyama coastal waters, Japan. Appl Environ Microbiol. 1989 Jun;55(6):1591–1598. doi: 10.1128/aem.55.6.1591-1598.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pee W., Stragier J. Evaluation of some cold enrichment and isolation media for the recovery of Yersinia enterocolitica. Antonie Van Leeuwenhoek. 1979;45(3):465–477. doi: 10.1007/BF00443284. [DOI] [PubMed] [Google Scholar]



