Abstract
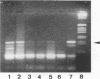
The application of the PCR to complex samples is hindered by amplification inhibitors. We describe a reverse transcription-PCR-based method capable of inhibitor removal for the detection of enteroviruses in shellfish. Initial virus extraction stages based on a modified polyethylene glycol precipitation technique (G.D. Lewis and T.G. Metcalf, Appl. Environ. Microbiol. 54:1983-1988, 1988) were followed by virus purification with 1,1,2-trichloro,2,2,1-trifluoroethane and concentration by ultrafiltration. A guanidine isothiocyanate-glass powder extraction system was utilized for sample lysis, RNase protection, and nucleic acid purification. Removal of PCR inhibitors and method sensitivity were quantified in shellfish (oysters and mussels) seeded with poliovirus. PCR sample tolerance exceeded 4 g for depurated shellfish; however, polluted field samples were more inhibitory. Virus recoveries of 31% for oyster extracts and 17% for mussel extracts and nucleic acid extraction reverse transcription-PCR detection limits down to 1 PFU yielded an overall sensitivity limit of < 10 PFU of poliovirus in up to 5 g of shellfish. PCR-positive results were obtained from a variety of polluted field samples naturally contaminated with human enteroviruses. The methods developed for virus recovery and PCR inhibitor removal should be equally applicable to detection of other RNA viruses such as hepatitis A virus, Norwalk virus, and other small round-structured viruses in shellfish.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton H. Foodborne viruses. Lancet. 1990 Dec 1;336(8727):1362–1364. doi: 10.1016/0140-6736(90)92905-w. [DOI] [PubMed] [Google Scholar]
- Atmar R. L., Metcalf T. G., Neill F. H., Estes M. K. Detection of enteric viruses in oysters by using the polymerase chain reaction. Appl Environ Microbiol. 1993 Feb;59(2):631–635. doi: 10.1128/aem.59.2.631-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce K. D., Hiorns W. D., Hobman J. L., Osborn A. M., Strike P., Ritchie D. A. Amplification of DNA from native populations of soil bacteria by using the polymerase chain reaction. Appl Environ Microbiol. 1992 Oct;58(10):3413–3416. doi: 10.1128/aem.58.10.3413-3416.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon R., Matsui S. M., Baric R. S., Herrmann J. E., Blacklow N. R., Greenberg H. B., Sobsey M. D. Detection of Norwalk virus in stool specimens by reverse transcriptase-polymerase chain reaction and nonradioactive oligoprobes. J Clin Microbiol. 1992 Dec;30(12):3151–3157. doi: 10.1128/jcm.30.12.3151-3157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill O. N., Cubitt W. D., McSwiggan D. A., Watney B. M., Bartlett C. L. Epidemic of gastroenteritis caused by oysters contaminated with small round structured viruses. Br Med J (Clin Res Ed) 1983 Nov 19;287(6404):1532–1534. doi: 10.1136/bmj.287.6404.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimåker M., Abebe A., Johansson B., Ehrnst A., Olcén P., Strannegård O. Detection of enteroviral RNA by polymerase chain reaction in faecal samples from patients with aseptic meningitis. J Med Virol. 1992 Sep;38(1):54–61. doi: 10.1002/jmv.1890380112. [DOI] [PubMed] [Google Scholar]
- Grohmann G. S., Murphy A. M., Christopher P. J., Auty E., Greenberg H. B. Norwalk virus gastroenteritis in volunteers consuming depurated oysters. Aust J Exp Biol Med Sci. 1981 Apr;59(Pt 2):219–228. doi: 10.1038/icb.1981.17. [DOI] [PubMed] [Google Scholar]
- Heller D., Gill O. N., Raynham E., Kirkland T., Zadick P. M., Stanwell-Smith R. An outbreak of gastrointestinal illness associated with consumption of raw depurated oysters. Br Med J (Clin Res Ed) 1986 Jun 28;292(6537):1726–1727. doi: 10.1136/bmj.292.6537.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill W. E., Keasler S. P., Trucksess M. W., Feng P., Kaysner C. A., Lampel K. A. Polymerase chain reaction identification of Vibrio vulnificus in artificially contaminated oysters. Appl Environ Microbiol. 1991 Mar;57(3):707–711. doi: 10.1128/aem.57.3.707-711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyypiä T., Auvinen P., Maaronen M. Polymerase chain reaction for human picornaviruses. J Gen Virol. 1989 Dec;70(Pt 12):3261–3268. doi: 10.1099/0022-1317-70-12-3261. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Stålhandske P., Vainionpä R., Pettersson U. Detection of enteroviruses by spot hybridization. J Clin Microbiol. 1984 Mar;19(3):436–438. doi: 10.1128/jcm.19.3.436-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. W., Siegl G., Lemon S. M. Molecular epidemiology of human hepatitis A virus defined by an antigen-capture polymerase chain reaction method. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2867–2871. doi: 10.1073/pnas.87.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang J., Graham D. Y., Estes M. K. Detection of Norwalk virus in stool by polymerase chain reaction. J Clin Microbiol. 1992 Oct;30(10):2529–2534. doi: 10.1128/jcm.30.10.2529-2534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Caul E. O., Ashley C. R., Clarke I. N. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science. 1993 Jan 22;259(5094):516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- Lewis G. D., Metcalf T. G. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol. 1988 Aug;54(8):1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. M., Kim J. P., Greenberg H. B., Su W., Sun Q., Johnson P. C., DuPont H. L., Oshiro L. S., Reyes G. R. The isolation and characterization of a Norwalk virus-specific cDNA. J Clin Invest. 1991 Apr;87(4):1456–1461. doi: 10.1172/JCI115152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf T. G., Moulton E., Eckerson D. Improved method and test strategy for recovery of enteric viruses from shellfish. Appl Environ Microbiol. 1980 Jan;39(1):141–152. doi: 10.1128/aem.39.1.141-152.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A. M., Grohmann G. S., Christopher P. J., Lopez W. A., Davey G. R., Millsom R. H. An Australia-wide outbreak of gastroenteritis from oysters caused by Norwalk virus. Med J Aust. 1979 Oct 6;2(7):329–333. doi: 10.5694/j.1326-5377.1979.tb104133.x. [DOI] [PubMed] [Google Scholar]
- Niederhauser C., Candrian U., Höfelein C., Jermini M., Bühler H. P., Lüthy J. Use of polymerase chain reaction for detection of Listeria monocytogenes in food. Appl Environ Microbiol. 1992 May;58(5):1564–1568. doi: 10.1128/aem.58.5.1564-1568.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive D. M., Al-Mufti S., Al-Mulla W., Khan M. A., Pasca A., Stanway G., Al-Nakib W. Detection and differentiation of picornaviruses in clinical samples following genomic amplification. J Gen Virol. 1990 Sep;71(Pt 9):2141–2147. doi: 10.1099/0022-1317-71-9-2141. [DOI] [PubMed] [Google Scholar]
- Picard C., Ponsonnet C., Paget E., Nesme X., Simonet P. Detection and enumeration of bacteria in soil by direct DNA extraction and polymerase chain reaction. Appl Environ Microbiol. 1992 Sep;58(9):2717–2722. doi: 10.1128/aem.58.9.2717-2722.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy B. L., Mackowiak P. A., Caraway C. T., Walker J. A., McKinley T. W., Klein C. A., Jr Oyster-associated hepatitis. Failure of shellfish certification programs to prevent outbreaks. JAMA. 1975 Sep 8;233(10):1065–1068. doi: 10.1001/jama.233.10.1065. [DOI] [PubMed] [Google Scholar]
- Power U. F., Collins J. K. Differential depuration of poliovirus, Escherichia coli, and a coliphage by the common mussel, Mytilus edulis. Appl Environ Microbiol. 1989 Jun;55(6):1386–1390. doi: 10.1128/aem.55.6.1386-1390.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart H. A. Enzymatic RNA amplification of the enteroviruses. J Clin Microbiol. 1990 Mar;28(3):438–442. doi: 10.1128/jcm.28.3.438-442.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobsey M. D., Carrick R. J., Jensen H. R. Improved methods for detecting enteric viruses in oysters. Appl Environ Microbiol. 1978 Jul;36(1):121–128. doi: 10.1128/aem.36.1.121-128.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. J., King R. K., Burchak J., Gannon V. P. Sensitive and specific detection of Listeria monocytogenes in milk and ground beef with the polymerase chain reaction. Appl Environ Microbiol. 1991 Sep;57(9):2576–2580. doi: 10.1128/aem.57.9.2576-2580.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Detection of low numbers of bacterial cells in soils and sediments by polymerase chain reaction. Appl Environ Microbiol. 1992 Feb;58(2):754–757. doi: 10.1128/aem.58.2.754-757.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. F., Cao W. W., Johnson M. G. 16S rRNA-based probes and polymerase chain reaction method to detect Listeria monocytogenes cells added to foods. Appl Environ Microbiol. 1992 Sep;58(9):2827–2831. doi: 10.1128/aem.58.9.2827-2831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernars K., Delfgou E., Soentoro P. S., Notermans S. Successful approach for detection of low numbers of enterotoxigenic Escherichia coli in minced meat by using the polymerase chain reaction. Appl Environ Microbiol. 1991 Jul;57(7):1914–1919. doi: 10.1128/aem.57.7.1914-1919.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernars K., Heuvelman C. J., Chakraborty T., Notermans S. H. Use of the polymerase chain reaction for direct detection of Listeria monocytogenes in soft cheese. J Appl Bacteriol. 1991 Feb;70(2):121–126. doi: 10.1111/j.1365-2672.1991.tb04437.x. [DOI] [PubMed] [Google Scholar]
- West P. A., Coleman M. R. A tentative national reference procedure for isolation and enumeration of Escherichia coli from bivalve molluscan shellfish by most probable number method. J Appl Bacteriol. 1986 Dec;61(6):505–516. doi: 10.1111/j.1365-2672.1986.tb01723.x. [DOI] [PubMed] [Google Scholar]
- Wilde J., Eiden J., Yolken R. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J Clin Microbiol. 1990 Jun;28(6):1300–1307. doi: 10.1128/jcm.28.6.1300-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde J., Van R., Pickering L., Eiden J., Yolken R. Detection of rotaviruses in the day care environment by reverse transcriptase polymerase chain reaction. J Infect Dis. 1992 Sep;166(3):507–511. doi: 10.1093/infdis/166.3.507. [DOI] [PubMed] [Google Scholar]
- Xi J. N., Graham D. Y., Wang K. N., Estes M. K. Norwalk virus genome cloning and characterization. Science. 1990 Dec 14;250(4987):1580–1583. doi: 10.1126/science.2177224. [DOI] [PubMed] [Google Scholar]
- Xu L., Harbour D., McCrae M. A. The application of polymerase chain reaction to the detection of rotaviruses in faeces. J Virol Methods. 1990 Jan;27(1):29–37. doi: 10.1016/0166-0934(90)90143-4. [DOI] [PubMed] [Google Scholar]
- Yamada O., Matsumoto T., Nakashima M., Hagari S., Kamahora T., Ueyama H., Kishi Y., Uemura H., Kurimura T. A new method for extracting DNA or RNA for polymerase chain reaction. J Virol Methods. 1990 Feb;27(2):203–209. doi: 10.1016/0166-0934(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Hironaka T., Kajiwara M., Tateno E., Kita H., Hirai K. Increased sensitivity for detection of human cytomegalovirus in urine by removal of inhibitors for the polymerase chain reaction. J Virol Methods. 1992 May;37(2):209–218. doi: 10.1016/0166-0934(92)90048-i. [DOI] [PubMed] [Google Scholar]
- Zhou Y. J., Estes M. K., Jiang X., Metcalf T. G. Concentration and detection of hepatitis A virus and rotavirus from shellfish by hybridization tests. Appl Environ Microbiol. 1991 Oct;57(10):2963–2968. doi: 10.1128/aem.57.10.2963-2968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mesquita M. M., Evison L. M., West P. A. Removal of faecal indicator bacteria and bacteriophages from the common mussel (Mytilus edulis) under artificial depuration conditions. J Appl Bacteriol. 1991 Jun;70(6):495–501. doi: 10.1111/j.1365-2672.1991.tb02746.x. [DOI] [PubMed] [Google Scholar]