Abstract
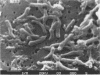
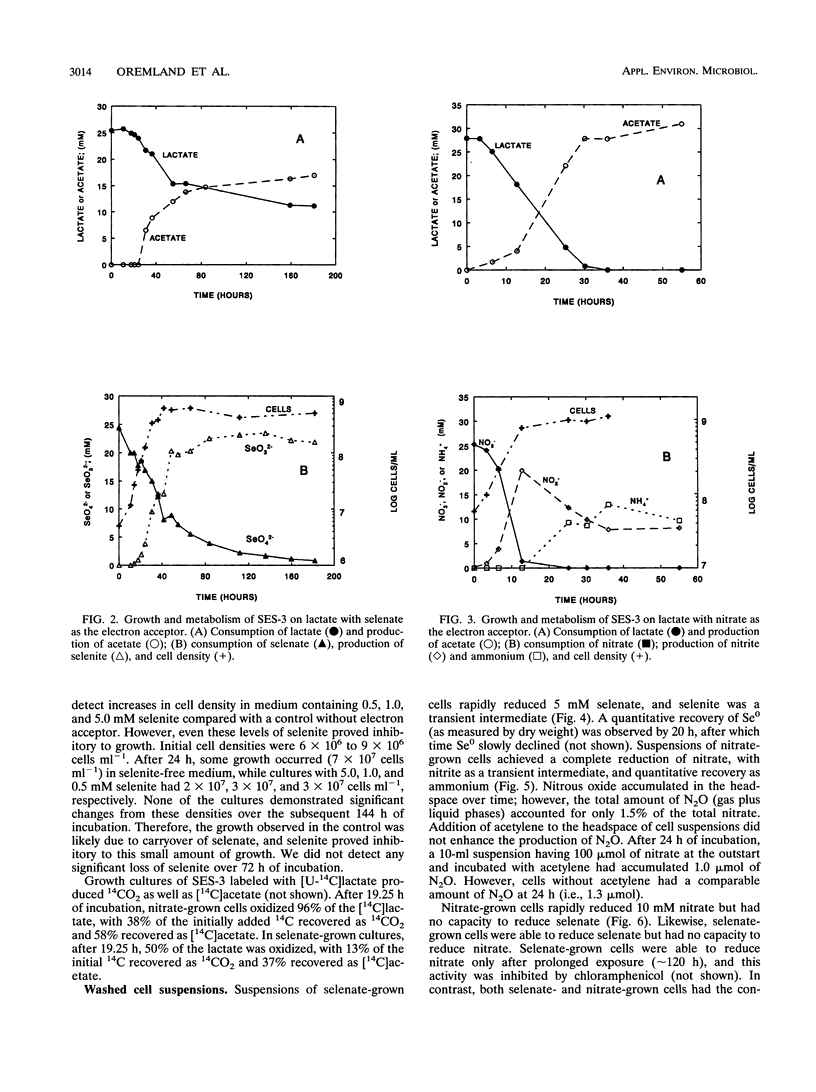
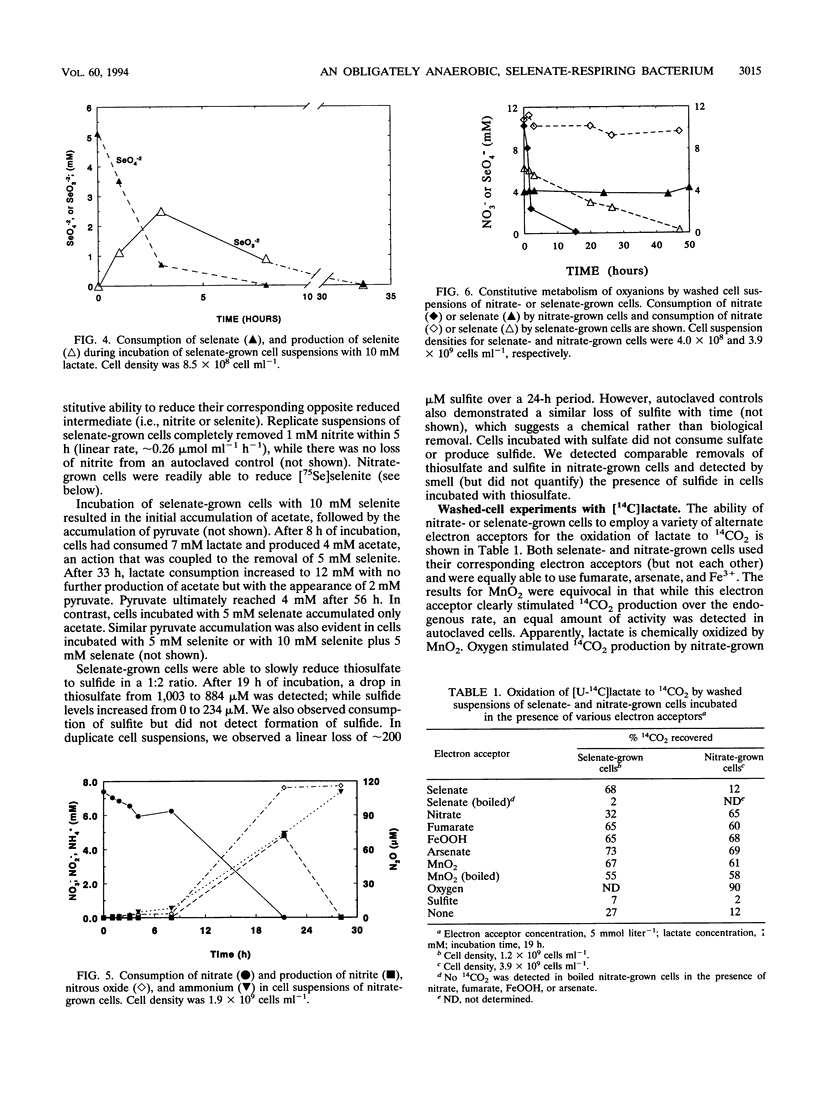
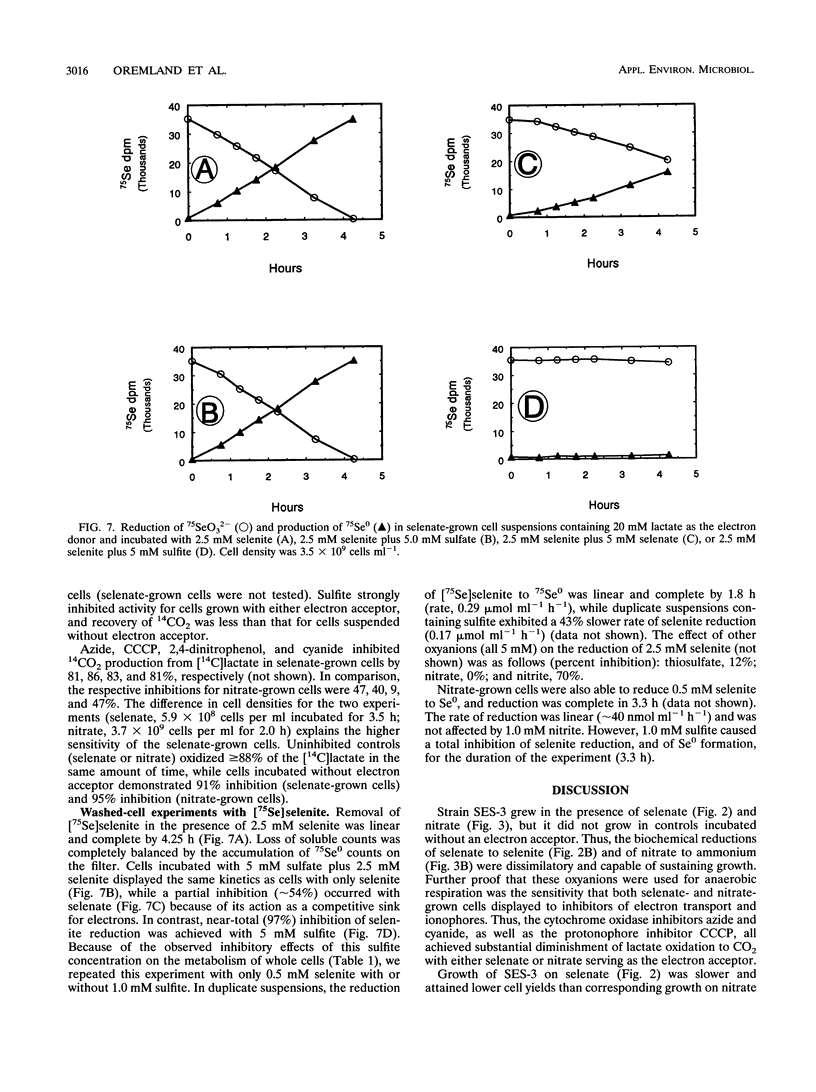
A gram-negative, strictly anaerobic, motile vibrio was isolated from a selenate-respiring enrichment culture. The isolate, designated strain SES-3, grew by coupling the oxidation of lactate to acetate plus CO2 with the concomitant reduction of selenate to selenite or of nitrate to ammonium. No growth was observed on sulfate or selenite, but cell suspensions readily reduced selenite to elemental selenium (Se0). Hence, SES-3 can carry out a complete reduction of selenate to Se0. Washed cell suspensions of selenate-grown cells did not reduce nitrate, and nitrate-grown cells did not reduce selenate, indicating that these reductions are achieved by separate inducible enzyme systems. However, both nitrate-grown and selenate-grown cells have a constitutive ability to reduce selenite or nitrite. The oxidation of [14C]lactate to 14CO2 coupled to the reduction of selenate or nitrate by cell suspensions was inhibited by CCCP (carbonyl cyanide m-chlorophenylhydrazone), cyanide, and azide. High concentrations of selenite (5 mM) were readily reduced to Se0 by selenate-grown cells, but selenite appeared to block the synthesis of pyruvate dehydrogenase. Tracer experiments with [75Se]selenite indicated that cell suspensions could achieve a rapid and quantitative reduction of selenite to Se0. This reduction was totally inhibited by sulfite, partially inhibited by selenate or nitrite, but unaffected by sulfate or nitrate. Cell suspensions could reduce thiosulfate, but not sulfite, to sulfide. These results suggest that reduction of selenite to Se0 may proceed, in part, by some of the components of a dissimilatory system for sulfur oxyanions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balderston W. L., Sherr B., Payne W. J. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol. 1976 Apr;31(4):504–508. doi: 10.1128/aem.31.4.504-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson C. W., Strohmaier F. E., Oremland R. S. Acetylene as a substrate in the development of primordial bacterial communities. Orig Life Evol Biosph. 1988;18(4):397–407. doi: 10.1007/BF01808218. [DOI] [PubMed] [Google Scholar]
- Culbertson C. W., Zehnder A. J., Oremland R. S. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol. 1981 Feb;41(2):396–403. doi: 10.1128/aem.41.2.396-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorby Y. A., Lovley D. R. Electron Transport in the Dissimilatory Iron Reducer, GS-15. Appl Environ Microbiol. 1991 Mar;57(3):867–870. doi: 10.1128/aem.57.3.867-870.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Tiedje J. M. Dissimilatory reduction of nitrate and nitrite in the bovine rumen: nitrous oxide production and effect of acetylene. Appl Environ Microbiol. 1981 Mar;41(3):705–709. doi: 10.1128/aem.41.3.705-709.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. F., Chadha R. K. Headspace liquid chromatographic technique for the determination of sulfite in food. J Chromatogr. 1987 Jul 10;398:355–359. doi: 10.1016/s0021-9673(01)96526-4. [DOI] [PubMed] [Google Scholar]
- Lortie L., Gould W. D., Rajan S., McCready R. G., Cheng K. J. Reduction of Selenate and Selenite to Elemental Selenium by a Pseudomonas stutzeri Isolate. Appl Environ Microbiol. 1992 Dec;58(12):4042–4044. doi: 10.1128/aem.58.12.4042-4044.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J., Lonergan D. J. Hydrogen and Formate Oxidation Coupled to Dissimilatory Reduction of Iron or Manganese by Alteromonas putrefaciens. Appl Environ Microbiol. 1989 Mar;55(3):700–706. doi: 10.1128/aem.55.3.700-706.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988 Jun;54(6):1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986 Apr;51(4):683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J. M., Michel T. A., Kirsch D. G. Selenate reduction by a Pseudomonas species: a new mode of anaerobic respiration. FEMS Microbiol Lett. 1989 Oct 1;52(1-2):195–198. doi: 10.1016/0378-1097(89)90195-x. [DOI] [PubMed] [Google Scholar]
- Macy J. M., Rech S., Auling G., Dorsch M., Stackebrandt E., Sly L. I. Thauera selenatis gen. nov., sp. nov., a member of the beta subclass of Proteobacteria with a novel type of anaerobic respiration. Int J Syst Bacteriol. 1993 Jan;43(1):135–142. doi: 10.1099/00207713-43-1-135. [DOI] [PubMed] [Google Scholar]
- Myers C. R., Nealson K. H. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science. 1988 Jun 3;240(4857):1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- Oremland R. S., Culbertson C. W. Evaluation of methyl fluoride and dimethyl ether as inhibitors of aerobic methane oxidation. Appl Environ Microbiol. 1992 Sep;58(9):2983–2992. doi: 10.1128/aem.58.9.2983-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Hollibaugh J. T., Maest A. S., Presser T. S., Miller L. G., Culbertson C. W. Selenate reduction to elemental selenium by anaerobic bacteria in sediments and culture: biogeochemical significance of a novel, sulfate-independent respiration. Appl Environ Microbiol. 1989 Sep;55(9):2333–2343. doi: 10.1128/aem.55.9.2333-2343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Steinberg N. A., Presser T. S., Miller L. G. In situ bacterial selenate reduction in the agricultural drainage systems of western Nevada. Appl Environ Microbiol. 1991 Feb;57(2):615–617. doi: 10.1128/aem.57.2.615-617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Umberger C., Culbertson C. W., Smith R. L. Denitrification in san francisco bay intertidal sediments. Appl Environ Microbiol. 1984 May;47(5):1106–1112. doi: 10.1128/aem.47.5.1106-1112.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech S. A., Macy J. M. The terminal reductases for selenate and nitrate respiration in Thauera selenatis are two distinct enzymes. J Bacteriol. 1992 Nov;174(22):7316–7320. doi: 10.1128/jb.174.22.7316-7320.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Annu Rev Biochem. 1990;59:111–127. doi: 10.1146/annurev.bi.59.070190.000551. [DOI] [PubMed] [Google Scholar]
- Steinberg N. A., Blum J. S., Hochstein L., Oremland R. S. Nitrate is a preferred electron acceptor for growth of freshwater selenate-respiring bacteria. Appl Environ Microbiol. 1992 Jan;58(1):426–428. doi: 10.1128/aem.58.1.426-428.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg N. A., Oremland R. S. Dissimilatory selenate reduction potentials in a diversity of sediment types. Appl Environ Microbiol. 1990 Nov;56(11):3550–3557. doi: 10.1128/aem.56.11.3550-3557.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr J. P., Oremland R. S. Reduction of selenate to selenide by sulfate-respiring bacteria: experiments with cell suspensions and estuarine sediments. Appl Environ Microbiol. 1987 Jun;53(6):1365–1369. doi: 10.1128/aem.53.6.1365-1369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]