Abstract
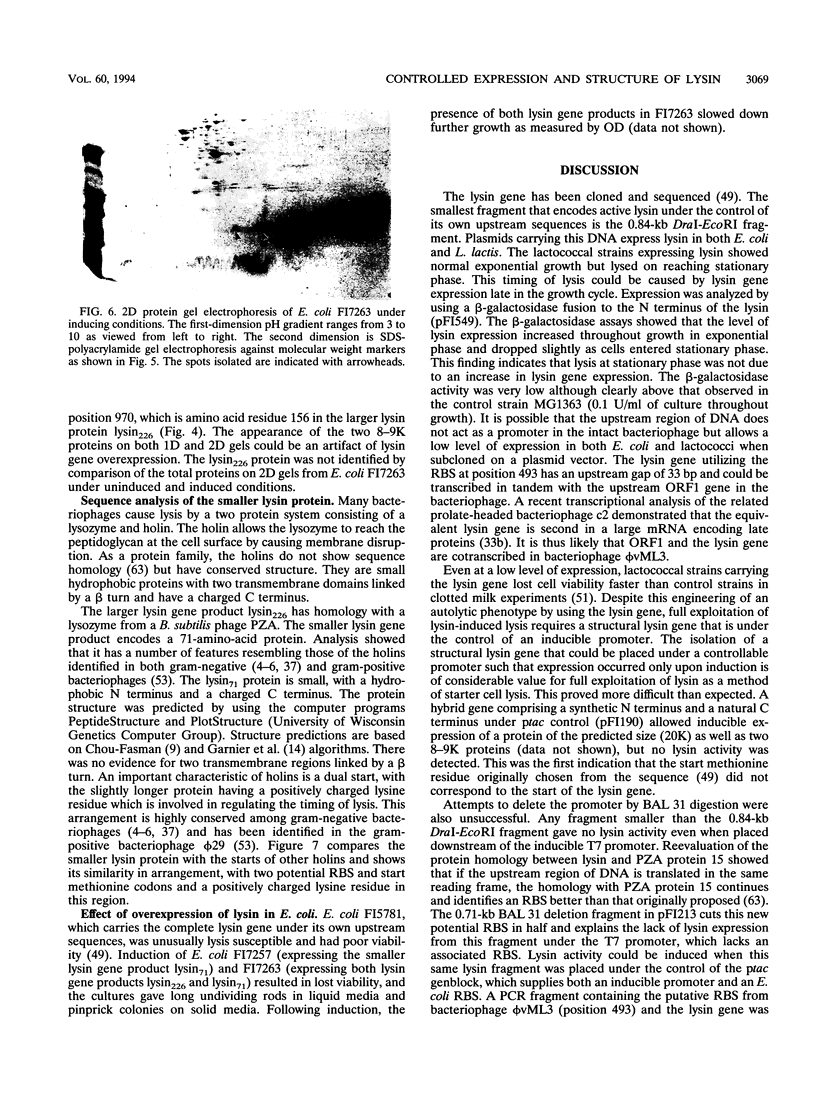
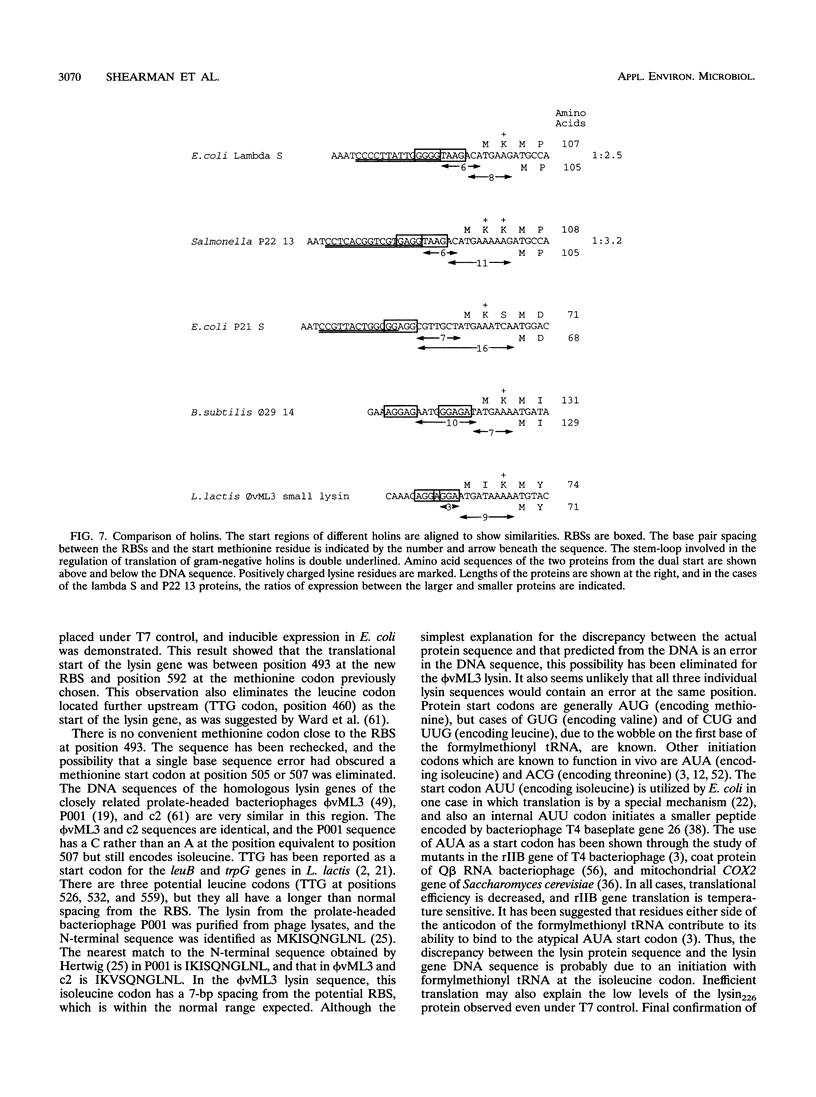
The phi vML3 bacteriophage lysin is specific for lactococci and could be used to promote enzyme release during cheese manufacture. The level of lysin expression from the cloned gene using its own upstream sequences is very low. Expression in Escherichia coli by using a synthetic hybrid lysin gene and a series of BAL 31 deletions of the original cloned DNA fragment suggested that the start of the gene had previously been incorrectly assigned. Reevaluation of homology between the lysin and Bacillus subtilis PZA protein 15 led to the identification of a new potential ribosome binding site (RBS). A 0.72-kb PCR-generated fragment including this RBS and the complete lysin gene was expressed and inducibly controlled. The translational start of the lysin gene was identified as an isoleucine codon, and this may lead to a low translation rate. During the analysis of the BAL 31 deletion fragments, two proteins of 20 and 8 kDa were shown to be expressed from the originally defined lysin gene. The DNA sequence has a second open reading frame with a good RBS and two potential start methionines. The smaller lysin protein was isolated, and the N terminus was sequenced, confirming that one methionine codon acted as the start of a second gene. The larger lysin protein has homology with lysozymes. The smaller lysin protein has some features resembling those of a holin. The possible roles of these two proteins in lysis of lactococci are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arendt E. K., Daly C., Fitzgerald G. F., van de Guchte M. Molecular characterization of lactococcal bacteriophage Tuc2009 and identification and analysis of genes encoding lysin, a putative holin, and two structural proteins. Appl Environ Microbiol. 1994 Jun;60(6):1875–1883. doi: 10.1128/aem.60.6.1875-1883.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardowski J., Ehrlich S. D., Chopin A. Tryptophan biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. 1992 Oct;174(20):6563–6570. doi: 10.1128/jb.174.20.6563-6570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D., Hedgpeth J., Selzer G. B., Epstein R. H. Temperature-sensitive mutation in the initiation codon of the rIIB gene of bacteriophage T4. Proc Natl Acad Sci U S A. 1979 Feb;76(2):700–704. doi: 10.1073/pnas.76.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläsi U., Chang C. Y., Zagotta M. T., Nam K. B., Young R. The lethal lambda S gene encodes its own inhibitor. EMBO J. 1990 Apr;9(4):981–989. doi: 10.1002/j.1460-2075.1990.tb08200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläsi U., Nam K., Hartz D., Gold L., Young R. Dual translational initiation sites control function of the lambda S gene. EMBO J. 1989 Nov;8(11):3501–3510. doi: 10.1002/j.1460-2075.1989.tb08515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonovich M. T., Young R. Dual start motif in two lambdoid S genes unrelated to lambda S. J Bacteriol. 1991 May;173(9):2897–2905. doi: 10.1128/jb.173.9.2897-2905.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Buzash-Pollert E., Studier F. W. Mutations of bacteriophage T7 that affect initiation of synthesis of the gene 0.3 protein. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2741–2745. doi: 10.1073/pnas.75.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Garrett J. M., Young R. Lethal action of bacteriophage lambda S gene. J Virol. 1982 Dec;44(3):886–892. doi: 10.1128/jvi.44.3.886-892.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J., Fusselman R., Hise J., Chiou L., Smith-Grillo D., Schulz J., Young R. Cell lysis by induction of cloned lambda lysis genes. Mol Gen Genet. 1981;182(2):326–331. doi: 10.1007/BF00269678. [DOI] [PubMed] [Google Scholar]
- Gasson M. J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godon J. J., Delorme C., Bardowski J., Chopin M. C., Ehrlich S. D., Renault P. Gene inactivation in Lactococcus lactis: branched-chain amino acid biosynthesis. J Bacteriol. 1993 Jul;175(14):4383–4390. doi: 10.1128/jb.175.14.4383-4390.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L., Stormo G., Saunders R. Escherichia coli translational initiation factor IF3: a unique case of translational regulation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7061–7065. doi: 10.1073/pnas.81.22.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz D., McPheeters D. S., Traut R., Gold L. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 1988;164:419–425. doi: 10.1016/s0076-6879(88)64058-4. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Holo H., Nes I. F. High-Frequency Transformation, by Electroporation, of Lactococcus lactis subsp. cremoris Grown with Glycine in Osmotically Stabilized Media. Appl Environ Microbiol. 1989 Dec;55(12):3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mulero J. J., Fox T. D. Reduced but accurate translation from a mutant AUA initiation codon in the mitochondrial COX2 mRNA of Saccharomyces cerevisiae. Mol Gen Genet. 1994 Feb;242(4):383–390. doi: 10.1007/BF00281787. [DOI] [PubMed] [Google Scholar]
- Nam K., Bläsi U., Zagotta M. T., Young R. Conservation of a dual-start motif in P22 lysis gene regulation. J Bacteriol. 1990 Jan;172(1):204–211. doi: 10.1128/jb.172.1.204-211.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivinskas R., Vaiskunaite R., Raudonikiene A. An internal AUU codon initiates a smaller peptide encoded by bacteriophage T4 baseplate gene 26. Mol Gen Genet. 1992 Mar;232(2):257–261. doi: 10.1007/BF00280004. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paces V., Vlcek C., Urbánek P., Hostomský Z. Nucleotide sequence of the right early region of Bacillus subtilis phage PZA completes the 19366-bp sequence of PZA genome. Comparison with the homologous sequence of phage phi 29. Gene. 1986;44(1):115–120. doi: 10.1016/0378-1119(86)90049-1. [DOI] [PubMed] [Google Scholar]
- Perry L. J., Heyneker H. L., Wetzel R. Non-toxic expression in Escherichia coli of a plasmid-encoded gene for phage T4 lysozyme. Gene. 1985;38(1-3):259–264. doi: 10.1016/0378-1119(85)90226-4. [DOI] [PubMed] [Google Scholar]
- Platteeuw C., de Vos W. M. Location, characterization and expression of lytic enzyme-encoding gene, lytA, of Lactococcus lactis bacteriophage phi US3. Gene. 1992 Sep 1;118(1):115–120. doi: 10.1016/0378-1119(92)90257-p. [DOI] [PubMed] [Google Scholar]
- Rennell D., Poteete A. R. Phage P22 lysis genes: nucleotide sequences and functional relationships with T4 and lambda genes. Virology. 1985 May;143(1):280–289. doi: 10.1016/0042-6822(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Saedi M. S., Garvey K. J., Ito J. Cloning and purification of a unique lysozyme produced by Bacillus phage phi 29. Proc Natl Acad Sci U S A. 1987 Feb;84(4):955–958. doi: 10.1073/pnas.84.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Shearman C., Underwood H., Jury K., Gasson M. Cloning and DNA sequence analysis of a Lactococcus bacteriophage lysin gene. Mol Gen Genet. 1989 Aug;218(2):214–221. doi: 10.1007/BF00331271. [DOI] [PubMed] [Google Scholar]
- Singer B. S., Gold L., Shinedling S. T., Colkitt M., Hunter L. R., Pribnow D., Nelson M. A. Analysis in vivo of translational mutants of the rIIB cistron of bacteriophage T4. J Mol Biol. 1981 Jul 5;149(3):405–432. doi: 10.1016/0022-2836(81)90479-4. [DOI] [PubMed] [Google Scholar]
- Steiner M., Lubitz W., Bläsi U. The missing link in phage lysis of gram-positive bacteria: gene 14 of Bacillus subtilis phage phi 29 encodes the functional homolog of lambda S protein. J Bacteriol. 1993 Feb;175(4):1038–1042. doi: 10.1128/jb.175.4.1038-1042.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlcek C., Paces V. Nucleotide sequence of the late region of Bacillus phage phi 29 completes the 19,285-bp sequence of phi 29 genome. Comparison with the homologous sequence of phage PZA. Gene. 1986;46(2-3):215–225. doi: 10.1016/0378-1119(86)90406-3. [DOI] [PubMed] [Google Scholar]
- Ward L. J., Beresford T. P., Lubbers M. W., Jarvis B. D., Jarvis A. W. Sequence analysis of the lysin gene region of the prolate lactococcal bacteriophage c2. Can J Microbiol. 1993 Aug;39(8):767–774. doi: 10.1139/m93-113. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. Bacteriophage lysis: mechanism and regulation. Microbiol Rev. 1992 Sep;56(3):430–481. doi: 10.1128/mr.56.3.430-481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]