Abstract
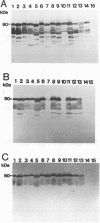
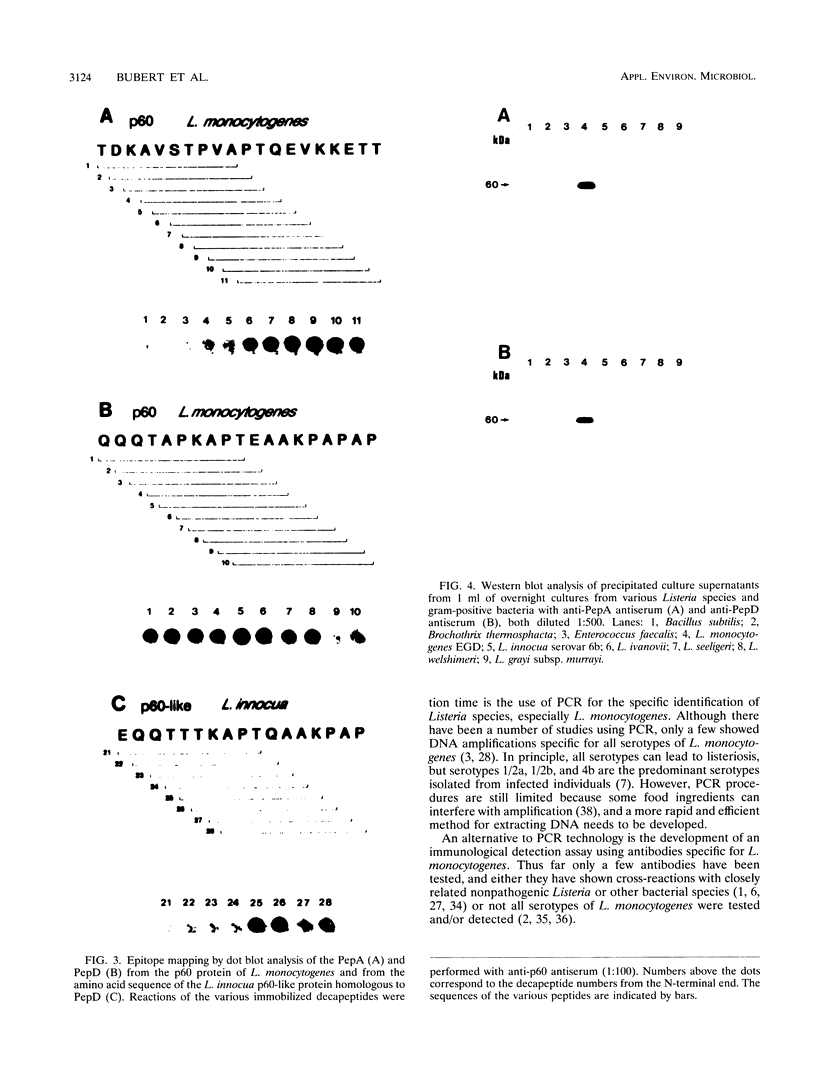
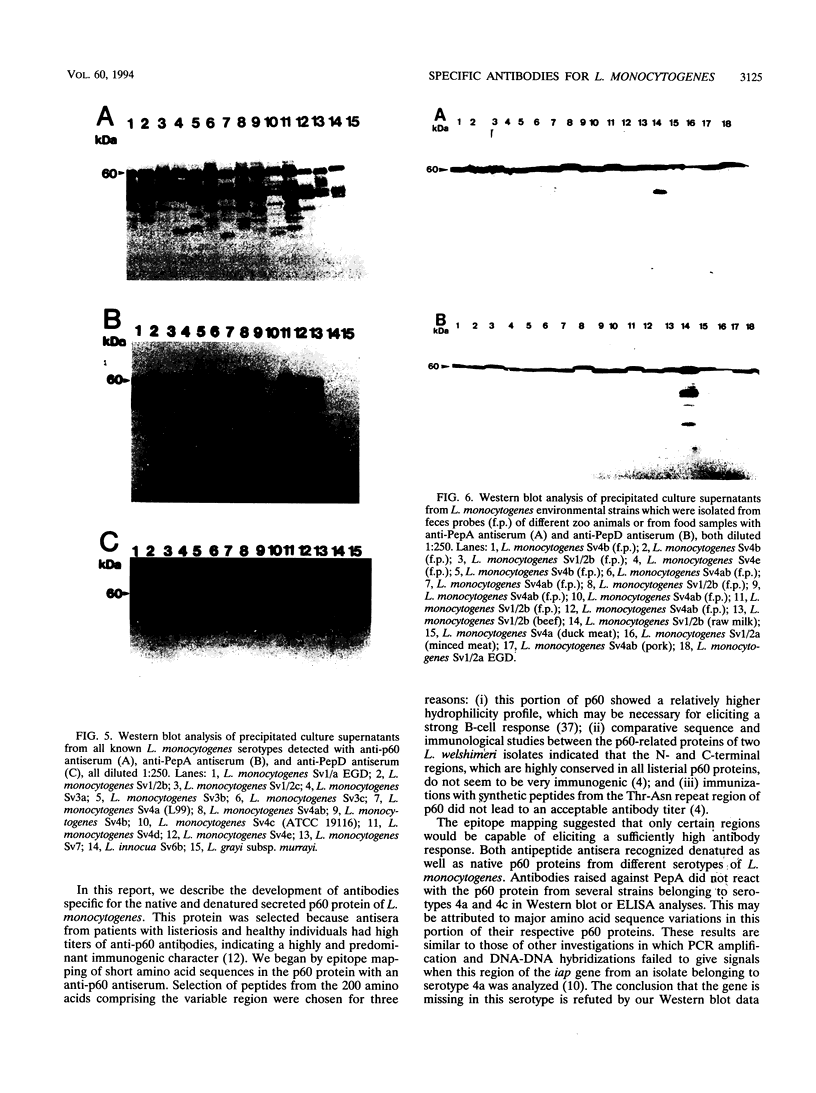
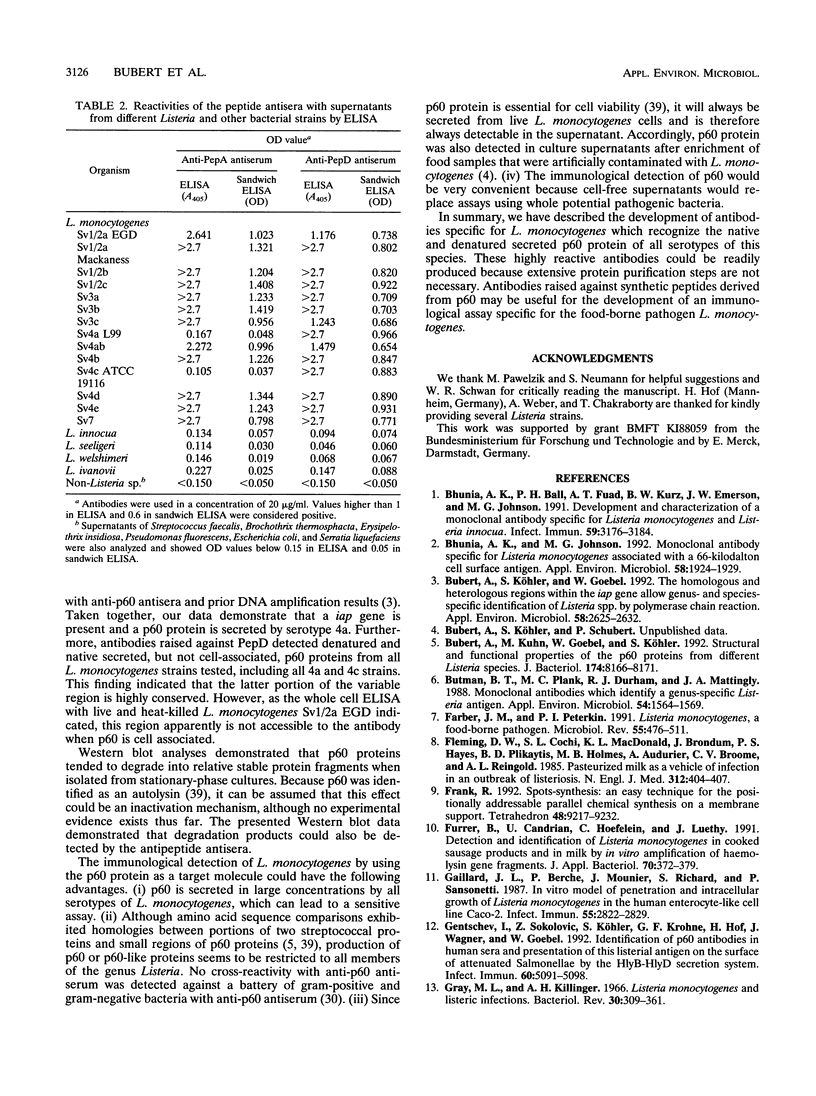
All species of the genus Listeria secrete a major extracellular protein called p60. A comparison of the deduced amino acid sequences of all listerial p60 proteins previously indicated there were only a few regions which were unique to the pathogenic, food-borne species Listeria monocytogenes. Two of these p60 regions were chosen for the development of antibodies specific for the facultative intracellular species L. monocytogenes. Initially, these regions were characterized via epitope mapping, and this led to the development of two different synthetic peptides. Rabbits immunized with these synthetic peptides generated polyclonal antibodies that were then used in Western blot (immunoblot) analyses. Antiserum against peptide A (PepA) recognized the p60 protein in the supernatants collected from most L. monocytogenes serotypes except for several strains belonging to serotypes 4a and 4c. No p60-related protein was detected in the supernatants from other Listeria species with this anti-PepA antiserum. Antibodies raised against peptide D (PepD) reacted with p60 from all L. monocytogenes serotypes, including all 4a and 4c strains that were tested, and also showed no cross-reactivity with supernatant proteins from other Listeria species. Both antisera also detected p60 in supernatants of a large number of environmental isolates of L. monocytogenes. Besides Western blot analyses, these antisera to PepA and PepD reacted with secreted p60 in an enzyme-linked immunosorbent assay, indicating recognition of the native antigen in addition to the denatured form. These data suggest that synthetic peptides derived from the variable region of the L. monocytogenes p60 protein may be useful for the development of an immunological diagnostic assay.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhunia A. K., Ball P. H., Fuad A. T., Kurz B. W., Emerson J. W., Johnson M. G. Development and characterization of a monoclonal antibody specific for Listeria monocytogenes and Listeria innocua. Infect Immun. 1991 Sep;59(9):3176–3184. doi: 10.1128/iai.59.9.3176-3184.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunia A. K., Johnson M. G. Monoclonal antibody specific for Listeria monocytogenes associated with a 66-kilodalton cell surface antigen. Appl Environ Microbiol. 1992 Jun;58(6):1924–1929. doi: 10.1128/aem.58.6.1924-1929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubert A., Kuhn M., Goebel W., Köhler S. Structural and functional properties of the p60 proteins from different Listeria species. J Bacteriol. 1992 Dec;174(24):8166–8171. doi: 10.1128/jb.174.24.8166-8171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubert A., Köhler S., Goebel W. The homologous and heterologous regions within the iap gene allow genus- and species-specific identification of Listeria spp. by polymerase chain reaction. Appl Environ Microbiol. 1992 Aug;58(8):2625–2632. doi: 10.1128/aem.58.8.2625-2632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butman B. T., Plank M. C., Durham R. J., Mattingly J. A. Monoclonal antibodies which identify a genus-specific Listeria antigen. Appl Environ Microbiol. 1988 Jun;54(6):1564–1569. doi: 10.1128/aem.54.6.1564-1569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming D. W., Cochi S. L., MacDonald K. L., Brondum J., Hayes P. S., Plikaytis B. D., Holmes M. B., Audurier A., Broome C. V., Reingold A. L. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985 Feb 14;312(7):404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- Furrer B., Candrian U., Hoefelein C., Luethy J. Detection and identification of Listeria monocytogenes in cooked sausage products and in milk by in vitro amplification of haemolysin gene fragments. J Appl Bacteriol. 1991 May;70(5):372–379. doi: 10.1111/j.1365-2672.1991.tb02951.x. [DOI] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentschev I., Sokolovic Z., Köhler S., Krohne G. F., Hof H., Wagner J., Goebel W. Identification of p60 antibodies in human sera and presentation of this listerial antigen on the surface of attenuated salmonellae by the HlyB-HlyD secretion system. Infect Immun. 1992 Dec;60(12):5091–5098. doi: 10.1128/iai.60.12.5091-5098.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. L., Killinger A. H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966 Jun;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
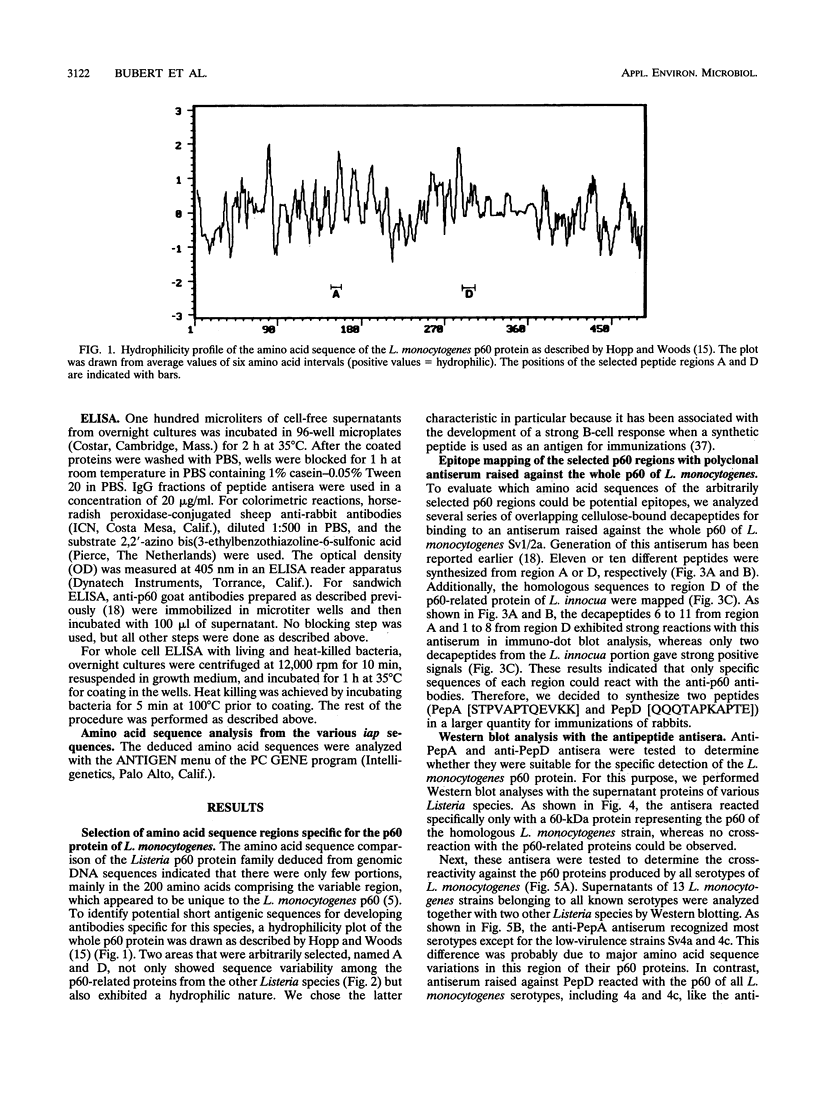
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Goebel W. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989 Jan;57(1):55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M., Kathariou S., Goebel W. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect Immun. 1988 Jan;56(1):79–82. doi: 10.1128/iai.56.1.79-82.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Köhler S., Bubert A., Vogel M., Goebel W. Expression of the iap gene coding for protein p60 of Listeria monocytogenes is controlled on the posttranscriptional level. J Bacteriol. 1991 Aug;173(15):4668–4674. doi: 10.1128/jb.173.15.4668-4674.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler S., Leimeister-Wächter M., Chakraborty T., Lottspeich F., Goebel W. The gene coding for protein p60 of Listeria monocytogenes and its use as a specific probe for Listeria monocytogenes. Infect Immun. 1990 Jun;58(6):1943–1950. doi: 10.1128/iai.58.6.1943-1950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loessner M. J. Improved procedure for bacteriophage typing of Listeria strains and evaluation of new phages. Appl Environ Microbiol. 1991 Mar;57(3):882–884. doi: 10.1128/aem.57.3.882-884.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly J. A., Butman B. T., Plank M. C., Durham R. J., Robison B. J. Rapid monoclonal antibody-based enzyme-linked immunosorbent assay for detection of Listeria in food products. J Assoc Off Anal Chem. 1988 May-Jun;71(3):679–681. [PubMed] [Google Scholar]
- Niederhauser C., Candrian U., Höfelein C., Jermini M., Bühler H. P., Lüthy J. Use of polymerase chain reaction for detection of Listeria monocytogenes in food. Appl Environ Microbiol. 1992 May;58(5):1564–1568. doi: 10.1128/aem.58.5.1564-1568.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Chakraborty T., Goebel W., Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992 Apr;60(4):1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhland G. J., Hellwig M., Wanner G., Fiedler F. Cell-surface location of Listeria-specific protein p60--detection of Listeria cells by indirect immunofluorescence. J Gen Microbiol. 1993 Mar;139(3):609–616. doi: 10.1099/00221287-139-3-609. [DOI] [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Lavigne P. M., Bortolussi R. A., Allen A. C., Haldane E. V., Wort A. J., Hightower A. W., Johnson S. E., King S. H., Nicholls E. S. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- Schuchat A., Swaminathan B., Broome C. V. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991 Apr;4(2):169–183. doi: 10.1128/cmr.4.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragusa G. R., Johnson M. G. Monoclonal antibody specific for Listeria monocytogenes, Listeria innocua, and Listeria welshimeri. Appl Environ Microbiol. 1990 Jun;56(6):1897–1904. doi: 10.1128/aem.56.6.1897-1904.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjerve E., Rørvik L. M., Olsvik O. Detection of Listeria monocytogenes in foods by immunomagnetic separation. Appl Environ Microbiol. 1990 Nov;56(11):3478–3481. doi: 10.1128/aem.56.11.3478-3481.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernars K., Heuvelman C. J., Chakraborty T., Notermans S. H. Use of the polymerase chain reaction for direct detection of Listeria monocytogenes in soft cheese. J Appl Bacteriol. 1991 Feb;70(2):121–126. doi: 10.1111/j.1365-2672.1991.tb04437.x. [DOI] [PubMed] [Google Scholar]
- Wuenscher M. D., Köhler S., Bubert A., Gerike U., Goebel W. The iap gene of Listeria monocytogenes is essential for cell viability, and its gene product, p60, has bacteriolytic activity. J Bacteriol. 1993 Jun;175(11):3491–3501. doi: 10.1128/jb.175.11.3491-3501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]