Abstract
We have developed mice deficient in membrane-bound dipeptidase (MBD, EC 3.4.13.19), the enzyme believed to be responsible for the conversion of leukotriene D4 (LTD4) to leukotriene E4 (LTE4). The MBD mutation generated by us was demonstrated to be a null mutation by Northern blot analysis and the absence of β-lactamase activity in lung, kidney, small intestine, and heart. MBD gene deletion had no effect on viability or fertility. The mutant mice retain partial ability to convert LTD4 to LTE4, ranging from 80–90% of the wild-type values in small intestine and liver to 16% in kidney and 40% in lung, heart, and pancreas. MBD is also believed to function consecutively after γ-glutamyl transpeptidase to cleave cystinyl-bis-glycine (cys-bis-gly) generated from glutathione cleavage. Our data indicate that kidney homogenates from MBD-deficient mice retain ∼40% of their ability to cleave cys-bis-gly, consistent with only modest elevations (3–5-fold) of cys-bis-gly in urine from MBD-deficient mice. These observations demonstrate that the conversion of LTD4 to LTE4 and the degradation of cys-bis-gly are catalyzed by at least two alternative pathways (one of which is MBD) that complement each other to varying extents in different tissues.
Keywords: glutathione eicosanoids, homologous recombination
Cysteinyl leukotrienes (LTC4, LTD4, and LTE4) are potent mediators of inflammatory responses and have been implicated in a number of acute and chronic inflammatory conditions (1–5). They are generated by the conjugation of the epoxide intermediate LTA4 with glutathione (GSH) to form LTC4 (6). This compound is degraded by the action of γ-glutamyl transpeptidase (GGT, EC 2.3.2.2) and the more recently characterized enzyme γ-glutamyl leukotrienase; these enzymes cleave the γ-glutamyl moiety from LTC4 to form the cysteinyl glycine conjugate of LTA4 (7, 8). LTD4 is then converted to LTE4 by a dipeptidase. The significance of this conversion is that LTE4 is 10- to 100-fold less active than LTD4 (3, 9–11). Hence, cleavage of LTD4 is an important step in the elimination of cysteinyl leukotriene function. Although it is by no means clear which dipeptidase(s) is/are involved in LTD4 to LTE4 catalysis, membrane-bound dipeptidase (MBD; dehydropeptidase-I, renal dipeptidase, microsomal dipeptidase; EC 3.4.13.19) has long been suspected to contribute to LTD4 inactivation (12–14).
In asthma, LTD4 and other cysteinyl leukotrienes induce and aggravate bronchoconstriction, vascular permeability, edema, and inflammation (1, 15–17). LTD4 is considered more potent than LTC4 in the induction of mucus formation and eosinophil accumulation (1, 18–20). LTD4 is also contributive to functional and structural changes during immune complex-induced inflammatory reactions in the glomerulus (21, 22). LTD4 is believed to induce and exacerbate ventricular arrhythmias and impair coronary flow and ventricular function (23, 24). In view of the potency of these compounds in responses to injury, it is of great importance to elucidate the regulation of their inactivation.
Glutathione (γ-glutamyl cysteinyl glycine) is the principal nonprotein thiol involved in a variety of cellular functions (25). Besides acting as a source of reducing equivalents in a number of biosynthetic reactions, it also plays a major role in the conjugation and metabolism of eicosanoids, toxins, and carcinogens (25, 26). GSH is metabolized extracellularly, principally in the renal tubules. The degradation of reduced and oxidized glutathione (GSH and GSSG) is initiated by GGT to form cysteinyl-glycine (cys-gly) and cystinyl-bis-glycine (cys-bis-gly) (11, 12). It is generally believed that cys-bis-gly is further metabolized into its constituent amino acids by MBD (11, 12, 25, 27). Because the majority of cys-gly in the renal tubules exists in the oxidized form, the recycling of GSH is postulated to be mediated via the consecutive actions of these two enzymes (12).
Although both GGT and MBD act in concert to metabolize a variety of compounds, their expression patterns are not concordant among various tissues. In tissues such as kidney and small intestine, they are both expressed at high levels, whereas in other tissues (fetal liver, lung, and seminal vesicles), one of the enzymes is absent or present at low levels. These findings suggest some functions that are concerted and others that are unrelated (28).
We have previously reported the genomic organization of MBD along with the comparison of the expression patterns of MBD and GGT (28). In the mouse, MBD is a single copy gene transcribed from at least three promoters that are used in a tissue-specific manner (ref. 28 and unpublished observations). To determine the physiological significance of MBD in the metabolism of leukotrienes, cys-bis-gly and cys-bis-gly conjugates of xenobiotics, we have generated mice carrying a null mutation in the MBD coding region. With the availability of MBD-deficient mice, we have now begun to address the issue of how different dipeptidases function in different tissues in the mouse.
MATERIALS AND METHODS
Generation of MBD-Deficient Mice.
A targeting vector for the MBD gene was constructed from the murine genomic clone isolated from a 129SvEv genomic library (Stratagene) (28). Fragments identical to their corresponding regions of the gene were inserted 5′ and 3′ of a phosphoglycerate kinase-hypoxanthine phosphoribosyltransferase expression cassette (29) such that the exon 1 coding region would be replaced by phosphoglycerate kinase-hypoxanthine phosphoribosyltransferase after homologous recombination of the plasmid with the endogenous locus (see Fig. 1A). The vector also contained an HSV-tk expression cassette 5′ of the insertion sites (30). The linearized targeting vector (25 μg) was electroporated into the HPRT-negative AB2.1 line of embryonic stem (ES) cells (107) and selected in hypoxanthine/aminopterin/thymidine (HAT) and 1-(2-deoxy-2-fluoro-β-arabinofuranosyl)-5-iodouracil (FIAU) as described (30). HATR, FIAUR ES cell clones were screened by Southern blot analysis (31) for correct targeting at the MBD locus. Germline transmission from chimeric males generated from three independent clones of ES cell lines was determined by Southern blot analysis as described (30). Mice were generated on C57BL/6/129SvEv hybrid and 129SvEv inbred genetic backgrounds. They were maintained 1–5 per cage and fed autoclaved Autoclavable Rodent Laboratory Chow 5010 from Purina Mills and water ad lib.
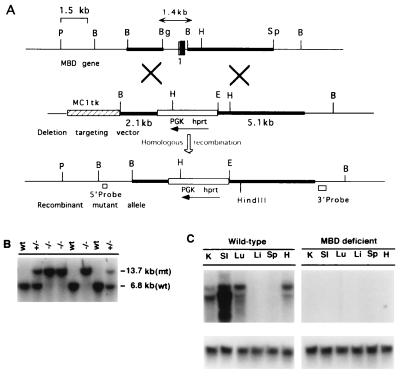
Figure 1.
Targeting strategy for homologous recombination at the MBD locus. (A) Partial restriction maps of the endogenous MBD gene, deletion targeting vector, and the recombinant mutant allele are shown. Coding sequences and introns that are replaced by homologous recombination are indicated by the following: vertical open box, noncoding exon of type I MBD RNA; vertical hatched box, common splice site necessary for all MBD RNAs; vertical solid box, part of the coding exon 1 containing the initiation site ATG and the introns (thin lines). Solid horizontal boxes represent the 5′ and 3′ arms. A phosphoglycerate kinase-hypoxanthine phosphoribosyltransferase expression cassette was used as the positive selectable marker, and an MC1tk expression cassette was used as a negative selectable marker as shown (31). B, BamHI; Bg, BglII; E, EcoRI; H, HindIII; P, PstI; Sp, SphI. (B) Southern blot analysis of BamHI-digested tail DNA; hybridization was performed using a 3′ external probe. wt, wild-type; +/−, heterozygote, −/−, homozygote. (C) Northern blot analysis of total RNA from various tissues of wild-type and MBD-deficient mice. The blots were hybridized using a 620-bp 32P-labeled mouse MBD cDNA probe corresponding to bases +610 to +1230 of the mouse MBD cDNA (28). The blot was stripped and reprobed with glyceraldehyde-3-phosphate dehydrogenase cDNA to verify equal loading in all lanes.
RNA Analysis by Northern Blotting.
Total RNAs were prepared from wild-type and MBD-deficient mice using the guanidinium thiocyanate procedure (32). Fifteen micrograms of total RNA from various tissues from wild-type and mice homozygous for the MBD mutation were electrophoresed on a 1% agarose, 2.2 M formaldehyde gel, transferred to a Zeta Probe nylon membrane (Bio-Rad), hybridized with a 620-bp 32P-labeled cDNA probe corresponding to the 3′-half of the mouse MBD cDNA (33), washed, and autoradiographed as described earlier (28).
β-Lactamase Assay.
β-lactamase activity (a specific measure of MBD activity) was assayed as previously described (34). Activity in homogenates from different tissues from wild-type, heterozygous, and homozygous mice was determined by measuring the rate of hydrolysis of the unsaturated dipeptide glycyldehydrophenylalanine. Reactions were carried out in quadruplicate.
LTD4 Conversion Assay.
Total tissue homogenates from wild-type and MBD- deficient male mice were incubated with 5 μM LTD4 in a total volume of 0.2 ml in 0.1 M Tris⋅HCl buffer, pH 8.0, for predetermined time intervals at 37°C. The reactions were terminated by the addition of 0.8 ml of methanol on ice for 10 min and centrifuged at 10,000 × g for 10 min. The supernatants were then purified on octadecyl disposable extraction column (J.T. Baker). LTD4 and its conversion products were eluted with methanol and evaporated in a Speedvac. The resuspended residues were injected onto a C18 reversed phase column (Customsil ODS, 4.6 × 150 mm, 3-μm particles, Custom LC, Houston) using the mobile phase methanol/water/acetic acid (65:35:0.1, pH 5.6, adjusted with NH4OH) (35). Specific activity of LTD4 conversion was expressed as nmol LTE4 formed/mg protein per h.
Cystinyl-bis-Glycine Metabolism.
Tissue homogenates from wild-type and MBD-deficient kidney were incubated with 0.4 mM cystinyl-bis-glycine in a total volume of 0.5 ml in 0.1 M Tris⋅HCl buffer, pH 8.0, at 37°C for different time intervals. The remaining cystinyl-bis-glycine and its conversion products were then incubated with 5 μl of 10 mM DTT to convert them to cysteinyl glycine and cysteine. The samples were derivatized with 2,4-dinitroflurobenzene and analyzed by reversed-phase ion exchange HPLC as described previously (8, 36).
RESULTS
Generation of MBD-Deficient Mice.
The MBD targeting vector was constructed using a clone isolated from a 129SvEv mouse genomic library (Fig. 1A). Fragments derived from the mouse MBD clone were used to prepare a plasmid that upon homologous recombination with the endogenous locus was predicted to result in the disruption of the gene for this protein. The targeting construct was designed to delete the regulatory region 5′ of exon 1, exon 1, and part of intron 1. Exon 1 contains the noncoding sequences for MBD type I RNA, a splice acceptor site necessary for the processing of the other three known MBD RNAs, a 16-amino acid signal peptide, and the first 18 amino acids of the mature MBD protein. This MBD fragment was replaced by a phosphoglycerate kinase-hypoxanthine phosphoribosyltransferase expression cassette (Fig. 1; also see refs. 28, 30, 37). As the signal peptide is required for the protein to be processed, absence of this sequence was expected to result in MBD deficiency. The construct was electroporated into AB2.1 cells (37). Resistant colonies were screened for the targeted (mutant) allele with both 5′ and 3′ external probes. Of 186 colonies screened with the 5′ external probe, 135 (73%) showed the expected 13-kb EcoRI recombinant mutant fragment indicative of the correctly targeted allele. We confirmed these results with a 3′ external probe and a BamHI digest; a 13.7-kb band diagnostic of the targeted mutant allele was identified (data not shown; see Fig. 1A). Three independent clones of ES cell lines were injected into blastocysts. All three led to the generation of male chimeras with ES cell-derived agouti coat pigmentation. A total of 95 agouti offspring were generated, and 45 (47%) were heterozygous for the mutant allele (MBDm1/+); this number agrees with the expected 50% transmission rate of the mutated allele. Heterozygous mice were viable and fertile. Heterozygous (MBDm1/+) F1 breeding pairs were set up using 14 male and 14 female heterozygotes, and their F2 offspring were genotyped by Southern blot analysis (Fig. 1B). MBDm1/MBDm1 mice were present in the litters in expected numbers based on Mendelian ratios for a heterozygous mating. This finding indicates that disruption of the MBD gene had no deleterious effect on fetal survival or development. The MBDm1/MBDm1 mice were indistinguishable visually from their MBD wild-type and MBD m1/+ littermates. We carried out extensive growth studies on MBDm1/MBDm1 mice along with wild-type littermates and found no difference in their growth rates on an 129SvEv/C57BL/6 background (data not shown). We also set up limited breeding pairs to obtain MBDm1/MBDm1 mice on 129SvEv background. These mice also seemed to grow at a normal rate. Both male and female MBDm1/MBDm1 mice are fertile.
Northern Analysis of MBD Expression in MBDm1/MBDm1 Mice.
Total RNA was isolated from six different tissues of wild-type and MBDm1/MBDm1 mice. A Northern blot probed with a mouse MBD 620-bp cDNA fragment (Fig. 1C) showed that murine MBD mRNA was present in the lanes corresponding to kidney, small intestine, lung, and heart in the MBD wild-type mice, but no hybridization was seen in the lanes corresponding to these organs in the MBDm1/MBDm1 mice. We also confirmed this finding by using an MBD cDNA probe containing sequences 3′ of those eliminated by targeting (data not shown). As we did not detect MBD mRNA, it is clear that a critical region of the MBD gene had been deleted through recombination.
MBDm1/MBDm1 Mice Do Not Hydrolyze β-Lactam Substrates.
MBD was initially identified and characterized as a mammalian β-lactamase and has been shown to catalyze the hydrolysis of a number of dipeptides, including penem and carbapenem derivatives (34, 38). To confirm that the MBDm1 was a null allele, we assayed mice for MBD β-lactam hydrolyzing activity (see Materials and Methods and Table 1) (34). In wild-type mice, MBD activity was high in lung and kidney and low in small intestine and heart. Activity was completely inhibitable by cilastatin, a known competitive inhibitor of MBD (39). Heterozygous (MBDm1/+) mice showed approximately half the activity of the wild-type mice. In MBDml/MBDml mice, we could not detect any MBD activity in lung, kidney, small intestine, or heart, the four organs in which MBD expression is highest (28). These results confirm that the MBDml is a null allele and that MBDml/MBDml mice completely lack MBD activity.
Table 1.
β-Lactamase activity in MBD-deficient mice
| Tissue | Wild type | Heterozygous | Homozygous |
|---|---|---|---|
| Lung | 1,530* | 579.8 | ND |
| Kidney | 996.5 | 431.4 | ND |
| Small intestine | 164.1 | 78.6 | ND |
| Heart | 147.6 | 75.4 | ND |
Glycyldehydrophenylalanine (70 μM) was used as the β-lactam substrate to assay MBD activity at 37°C using 100 μg of protein (34). Each determination was performed in quadruplicate, and at least three mice were used. Each SEM was 5-15% of the averaged values. ND, no detectable activity.
nmol glycyldehydrophenylalanine cleaved/mg protein/h at 37°C.
LTD4 Cleavage in MBD-Deficient Mice.
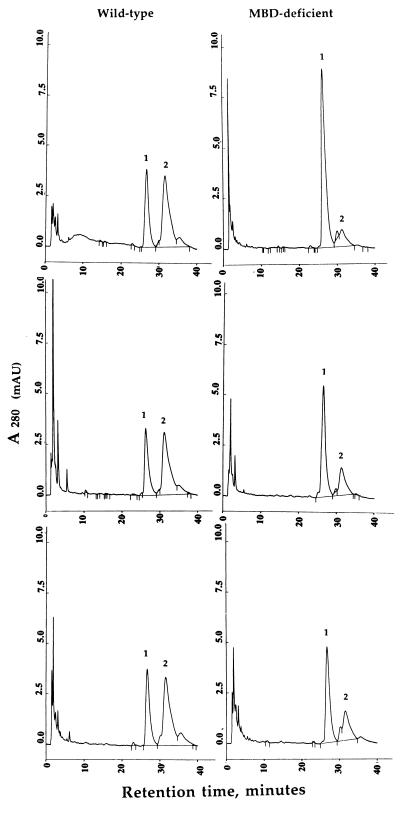
Because LTD4 conversion to LTE4 is thought to be an MBD-mediated event, we analyzed this reaction in MBD-deficient mice. Our initial experiments with kidney homogenates from wild-type mice showed that 100 μg of protein convert 60% of the LTD4 to LTE4 in 30 min; in contrast, extracts from MBD-deficient kidney converted only 12% of LTD4 to LTE4 (see Fig. 2 Top). We confirmed these observations by assaying other tissues in which MBD expression is known to be high. When we incubated lung homogenates from wild-type mice with LTD4, approximately 60% was converted to LTE4 with 50 μg of protein in 30 min. Under identical conditions, lung extracts from MBD-deficient mice converted 25% of the LTD4 to LTE4 (Fig. 2 Middle). Heart homogenates from wild-type mice convert 62% of LTD4 to LTE4 in 75 min, whereas homogenates from MBD-deficient mice cleave 30% of LTD4 to LTE4 (Fig. 2 Bottom). Thus, MBD-deficient mice retain substantial ability to metabolize LTD4 to LTE4.
Figure 2.
Analysis of LTD4 metabolism by tissue homogenates of kidney, lung, and heart of wild-type (Left) and MBD-deficient (Right) mice by HPLC. The reaction conditions are as described under Materials and Methods. The peaks labeled as 1 and 2 refer to LTD4 and LTE4, respectively. For kidney (Top), 100 μg of protein and an incubation time of 30 min was used. Lung extracts were assayed using 50 μg of protein for 30 min (Middle). Heart extracts were incubated for 75 min and contained 100 μg of protein (Bottom).
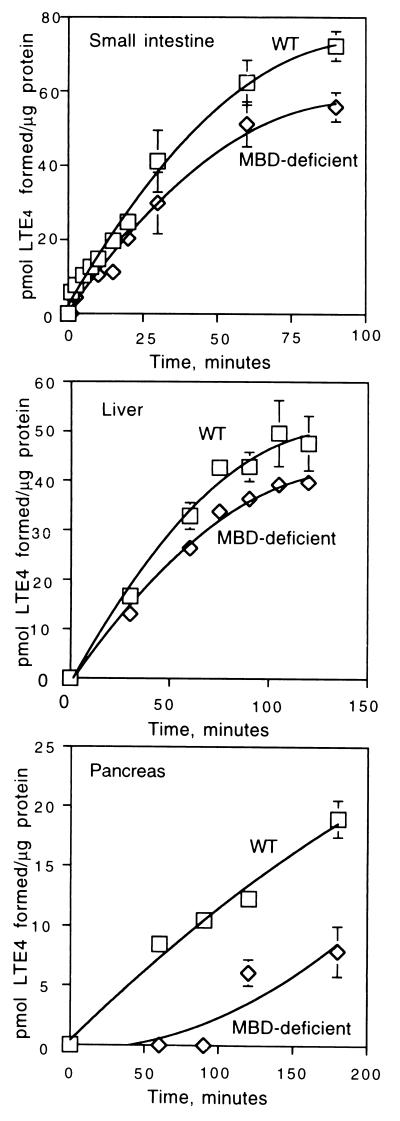
To develop more quantitative comparisons, we have evaluated LTD4 to LTE4 converting activity in a variety of tissues (Fig. 3). Results from these and similar experiments were used to determine the specific activity of LTD4 to LTE4 conversion from different tissues of wild-type and MBD-deficient mice (Table 2). In wild-type mice, the highest activity was found in lung, followed by kidney and small intestine. Heart, liver, and pancreas had lower levels. In MBD-deficient mice, the enzyme(s) responsible for LTD4 cleavage seem to have similar specific activities in lung and small intestine. Kidney and liver from MBD-deficient mice express this activity at about 25–35% of the levels found in lung, whereas the levels in heart and pancreas are somewhat lower. Thus, in small intestine where MBD expression is relatively high (Table 1), its contribution to LTD4 clearance is low (Table 2), whereas in lung in which MBD activity is also high (Table 1), it accounts for about two-thirds of the LTD4 conversion to LTE4 (Table 2).
Figure 3.
Time course of LTD4 metabolism by small intestine, liver, and pancreas of wild-type and MBD-deficient mice. Total homogenates from these tissues were assayed for LTD4 to LTE4 conversion as described under Materials and Methods. For all assays, 100 μg of protein was used. Squares indicate wild-type and diamonds denote MBD-deficient assays. All assays were carried out at 37°C.
Table 2.
LTD4 dipeptidase activity in wild-type and MBD-deficient mice
| Tissue | Wild type | MBD-deficient | Wild-type activity†, % |
|---|---|---|---|
| Lung | 247.5* | 86.6 | 35 |
| Kidney | 179.6 | 28.6 | 16 |
| Small intestine | 101.2 | 87.2 | 86 |
| Heart | 39.4 | 12.1 | 31 |
| Liver | 32.8 | 26.4 | 81 |
| Pancreas | 8.5 | 3.1 | 37 |
Assays were performed in duplicate by using tissue homogenates from male mice. For each determination, three mice were used. For calculation of specific activity, values from the initial portion of the curve where the activity was most linear were used. Each SEM was 10-20% of the averaged values.
nmol LTE4 formed/mg protein per h.
MBD-deficient specific activity/Wild-type specific activity × 100.
Assessment of cys-bis-gly Cleavage in MBD-Deficient Mice.
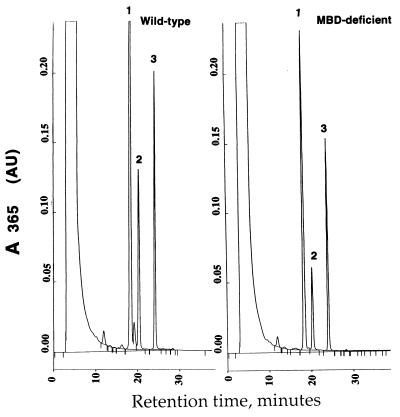
We have previously shown that GGT-deficient mice fail to thrive because of a cysteine deficiency resulting from urinary loss of cysteine as uncleaved GSH (37). However, unlike GGT-deficient mice, which show ∼2,500-fold higher levels of GSH in the urine, MBD-deficient mice have only a 3- to 5-fold elevation of urinary cys-bis-gly (7.9 μM in wild-type versus 33.8 μM in MBD-deficient mice). MBD has been implicated as the enzyme responsible for cleavage of cys-bis-gly to cysteine and glycine (11, 27). We adapted a reversed phase ion-exchange HPLC method used to separate GSH and GSSG (36) to examine cys-bis-gly cleavage to cysteine. In the assay, we used DTT to convert uncleaved substrate cys-bis-gly and product cystine to cys-gly and cysteine, respectively. We monitored the appearance of one of the cleavage products (cysteine) spectrophotometrically. The results from these experiments indicate that the kidney homogenates from MBD-deficient mice cleave cys-bis-gly at a reduced rate of 40% compared with wild-type kidney homogenates (Fig. 4). These observations indicate that there are other enzymes in addition to MBD capable of converting cys-bis-gly to cysteine.
Figure 4.
Analysis of cys-bis-gly metabolism in kidney homogenates from wild-type and MBD-deficient mice. For details on assay conditions and sample preparation, see Materials and Methods. (A) HPLC profile of cys-bis-gly metabolism by homogenates of wild-type kidney. The peaks labeled 1, 2, and 3 represent cys-gly, cysteine, and the internal standard γ-glu-glu, respectively. (B) HPLC profile of cys-bis-gly metabolism in kidney homogenates from MBD-deficient mice.
DISCUSSION
We have developed mice with a null mutation at the MBD locus (Fig. 1, Table 1). These mice grow and develop normally and are fertile. Our results also confirm that β-lactam hydrolysis is a specific assay for MBD in the mouse (Table 1).
Although MBD exhibits activity toward a variety of dipeptide substrates, the biological role of this enzyme is not well understood. Two principal physiological functions have been attributed to MBD: 1) the conversion of LTD4 to LTE4 and 2) the metabolism of cys-bis-gly and cys-bis-gly conjugates of toxins and xenobiotics. Our studies indicate that MBD is not the only enzyme capable of these functions (Table 2, Figs. 2–4). MBD is a major contributor to LTD4 conversion activity in kidney, lung, heart, and pancreas, but in tissues like small intestine and liver, MBD seems to play a minor role. We have assayed activity at pH 8.0 in 0.1 M Tris⋅HCl buffer, conditions optimal for MBD. Because these conditions may not be optimal for other converting enzymes, the percent contribution of MBD to LTD4 conversion should be viewed as approximate.
It is unclear why more than one LTD4 converting enzyme is necessary. One explanation is that the action of LTD4 is life-threatening if left unchecked, and consequently dual systems may have evolved. Another possibility is that MBD and other enzymes function in different local environments (28). As for the high level of LTD4 converting activity in kidney attributable to MBD (Table 2), the most likely explanation is that it represents a “by-product” of MBD’s role in GSH metabolism. This idea is supported by our previous demonstration of high levels of expression of MBD in the proximal convoluted tubules (28). Similarly, MBD is expressed at high levels in the villous epithelium of the small intestine, where it is more likely to participate in GSH metabolism (ref. 11 and unpublished observations). The fact that only about 14% of the LTD4 converting activity in the small intestine is attributable to MBD again supports the idea that other enzyme(s) can catalyze this process.
MBD-deficient mice do not share any of the stigmata of GGT-deficient mice (37). They grow and mature normally and are not cysteine-deficient. The most likely explanation for the lack of cysteine deficiency is that MBD accounts for only 40–50% of the cys-bis-gly cleaving activity in kidney. Consequently, there is only moderate loss of cysteine as cys-bis-gly in the urine. Although we have not examined the hydrolysis of any S-cys-gly conjugates (xenobiotics or carcinogens) in MBD-deficient mice, it is unlikely given our findings with LTD4 metabolism that MBD is the only enzyme capable of such cleavage. Our findings are also consistent with a report of LTD4 to LTE4 conversion by lysosomal enzymes from human polymorphonuclear leukocytes; however, it is unknown if this enzyme(s) represents the residual activity we have detected (40).
Our results, along with the characterization of a newly identified enzyme γ-glutamyl leukotrienase (8), demonstrate that cysteinyl leukotriene metabolism is more complex than previously believed. The role of MBD and other enzymes that cleave LTD4 as well as the contribution of GGT and γ-glutamyl leukotrienase to leukotriene metabolism remain to be explored.
Acknowledgments
We thank Dr. C.-N. Ou for helpful discussions on HPLC and Jinwen Dong, Cheryl L. Rognerud, and Amy L. Wiseman for technical assistance. This work was supported by National Institutes of Health Grant ES 08668 (M.W.L).
ABBREVIATIONS
- cys-bis-gly
cystinyl-bis-glycine
- cys-gly
cysteinyl glycine
- GSH
glutathione
- GGT
γ-glutamyl transpeptidase
- γ-glu-glu
γ-glutamyl glutamate
- LTD4
leukotriene D4
- LTE4
leukotriene E4
- MBD
membrane-bound dipeptidase
- ES
embryonic stem
References
- 1.Henderson W R., Jr Ann Intern Med. 1994;121:684–697. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- 2.Lewis R A, Austen K F, Soberman R J. N Engl J Med. 1990;323:645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 3.O’Byrne P M. Ann NY Acad Sci. 1994;744:251–261. doi: 10.1111/j.1749-6632.1994.tb52743.x. [DOI] [PubMed] [Google Scholar]
- 4.Fauler J, Neumann C, Tsikas D, Frolich J C. J Invest Dermatol. 1992;99:8–11. doi: 10.1111/1523-1747.ep12611380. [DOI] [PubMed] [Google Scholar]
- 5.Fauler J, Thon A, Tsikas D, von der Hardt H, Frolich J C. Arthritis Rheum. 1994;37:93–97. doi: 10.1002/art.1780370114. [DOI] [PubMed] [Google Scholar]
- 6.Penrose J F, Gagnon L, Goppelt-strube M, Myers P, Lam B K, Jack R M, Austen K F, Soberman R J. Proc Natl Acad Sci USA. 1992;89:11603–11606. doi: 10.1073/pnas.89.23.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis R A, Austen K F. In: Inflammation: Basic Principles and Clinical Correlates. Gallin J I, Goldstein I M, Snyderman R, editors. New York: Raven; 1988. pp. 121–128. [Google Scholar]
- 8.Carter B Z, Wiseman A L, Orkiszewski R, Ballard K D, Ou C-N, Lieberman M W. J Biol Chem. 1997;272:12305–12310. doi: 10.1074/jbc.272.19.12305. [DOI] [PubMed] [Google Scholar]
- 9.Dahlen S E, Hedqvist P, Hammarstrom S, Samuelsson B. Nature (London) 1980;288:484–486. doi: 10.1038/288484a0. [DOI] [PubMed] [Google Scholar]
- 10.Hanna C J, Bach M K, Pare P D, Schallenberg R R. Nature (London) 1981;290:343–344. doi: 10.1038/290343a0. [DOI] [PubMed] [Google Scholar]
- 11.Kozak E M, Tate S S. J Biol Chem. 1982;257:6322–6327. [PubMed] [Google Scholar]
- 12.Curthoys N P. In: Glutathione: Metabolism and Physiological Functions. Vina J, editor. Boca Raton, FL: CRC; 1990. pp. 217–225. [Google Scholar]
- 13.Adachi H, Kubota I, Okamura N, Iwata H, Tsujimoto M, Nakazato H, Nichihara T, Noguchi T. J Biochem (Tokyo) 1989;105:957–961. doi: 10.1093/oxfordjournals.jbchem.a122787. [DOI] [PubMed] [Google Scholar]
- 14.Huber M, Keppler D. Eur J Biochem. 1987;167:73–79. doi: 10.1111/j.1432-1033.1987.tb13305.x. [DOI] [PubMed] [Google Scholar]
- 15.Lewis R A, Austen K F. J Clin Invest. 1984;73:889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson W R., Jr Ann Allergy. 1994;72:272–278. [PubMed] [Google Scholar]
- 17.Dahlen S E, Hansson G, Hedqvist P T, Bjorck E, Granstrom E, Dahlen B. Proc Natl Acad Sci USA. 1983;80:1712–1716. doi: 10.1073/pnas.80.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson W R, Jr, Lewis D B, Albert R K, Zhang Y, Lamm W J E, Chiang G K S, Jones F, Eriksen P, Tien Y-T, Jonas M, Chi E Y. J Exp Med. 1996;184:1483–1494. doi: 10.1084/jem.184.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laitinen L A, Laitinen A, Haahtela T, Vilkka V, Spur B W, Lee T H. Lancet. 1993;341:989–990. doi: 10.1016/0140-6736(93)91073-u. [DOI] [PubMed] [Google Scholar]
- 20.Spada C S, Nieves A L, Krauss A H-P, Woodward D F. J Leukocyte Biol. 1994;55:183–191. doi: 10.1002/jlb.55.2.183. [DOI] [PubMed] [Google Scholar]
- 21.Petric R, Ford-Hutchinson A W. Kidney Int. 1994;46:1322–1329. doi: 10.1038/ki.1994.401. [DOI] [PubMed] [Google Scholar]
- 22.Nassar G M, Badr K F. Miner Electrolyte Metab. 1995;21:262–270. [PubMed] [Google Scholar]
- 23.Lee C C, Appleyard R F, Byrne J G, Cohn L H. Cardiovasc Res. 1993;27:770–773. doi: 10.1093/cvr/27.5.770. [DOI] [PubMed] [Google Scholar]
- 24.Carry M, Korley V, Willerson J T, Weigelt L, Ford-Hutchinson A W, Tagari P. Circulation. 1991;85:230–236. doi: 10.1161/01.cir.85.1.230. [DOI] [PubMed] [Google Scholar]
- 25.Meister A, Larsson A. In: The Metabolic Basis of Inherited Disease. 7th Ed. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 1461–1477. [Google Scholar]
- 26.Lieberman M W, Barrios R, Carter B Z, Habib G M, Lebovitz R M, Rajagopalan S, Sepulveda A R, Shi Z-Z, Wan D-F. Am J Pathol. 1995;147:1175–1185. [PMC free article] [PubMed] [Google Scholar]
- 27.Hirota T, Nishikawa Y, Tanaka M, Igrashi T, Kitagawa H. Eur J Biochem. 1986;160:521–525. doi: 10.1111/j.1432-1033.1986.tb10070.x. [DOI] [PubMed] [Google Scholar]
- 28.Habib G M, Barrios R, Shi Z-Z, Lieberman M W. J Biol Chem. 1996;271:16273–16280. doi: 10.1074/jbc.271.27.16273. [DOI] [PubMed] [Google Scholar]
- 29.Matzuk M M, Finegold M J, Su J-G J, Hsueh A J W, Bradley A A. Nature (London) 1992;360:313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- 30.Mansour S L, Thomas K R, Capecchi M R. Nature (London) 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez-Solis R, Rivera-Perez J, Wallace J D, Wims M, Zheng H, Bradley A A. Anal Biochem. 1992;201:331–335. doi: 10.1016/0003-2697(92)90347-a. [DOI] [PubMed] [Google Scholar]
- 32.Chomczynski P, Sachi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 33.Satoh S, Keida Y, Konta Y, Maeda M, Matsumoto Y, Niwa M, Kohsaka M. Biochim Biophys Acta. 1993;1163:234–242. doi: 10.1016/0167-4838(93)90157-m. [DOI] [PubMed] [Google Scholar]
- 34.Kropp, H., Sundelof, J. G., Hajdu, R. & Kahan, F. M. (1982) J. Antimicrob. Chemother. 22, Suppl. D., 62–70. [DOI] [PMC free article] [PubMed]
- 35.Denzlinger C, Guhlmann A, Schueber P H, Wilker D, Hammer D K, Keppler D. J Biol Chem. 1986;261:15601–15606. [PubMed] [Google Scholar]
- 36.Fariss M W, Reed D J. Methods Enzymol. 1987;143:101–109. doi: 10.1016/0076-6879(87)43018-8. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman M W, Wiseman A L, Shi Z-Z, Carter B Z, Barrios R, Ou C-N, Chevez-Barrios P, Wang Y, Habib G M, Goodman J C, et al. Proc Natl Acad Sci USA. 1996;93:7923–7926. doi: 10.1073/pnas.93.15.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H S, Campbell B J. Biochem Biophys Res Commun. 1982;108:1638–1642. doi: 10.1016/s0006-291x(82)80097-1. [DOI] [PubMed] [Google Scholar]
- 39.Campbell B J, Forrester L J, Zahler W L, Burks M. J Biol Chem. 1984;259:14586–14590. [PubMed] [Google Scholar]
- 40.Lee C W, Lewis R A, Corey E J, Austen K F. Immunology. 1983;48:27–35. [PMC free article] [PubMed] [Google Scholar]