Abstract
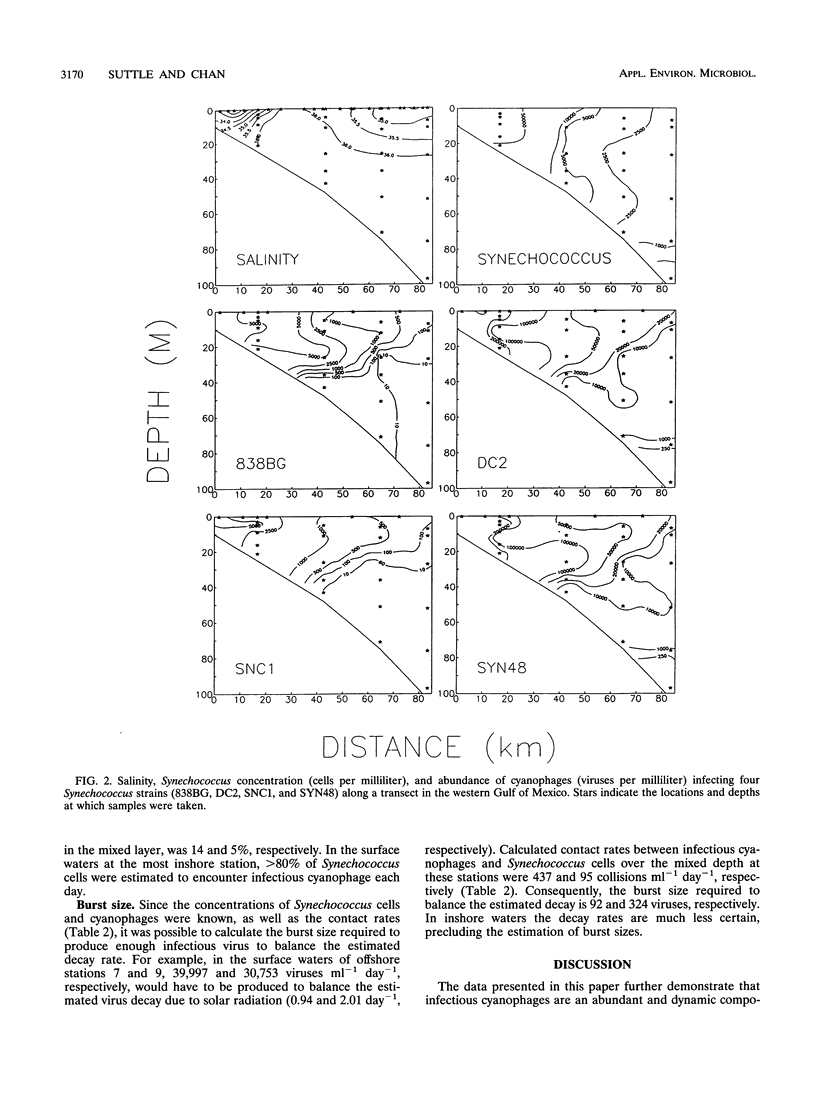
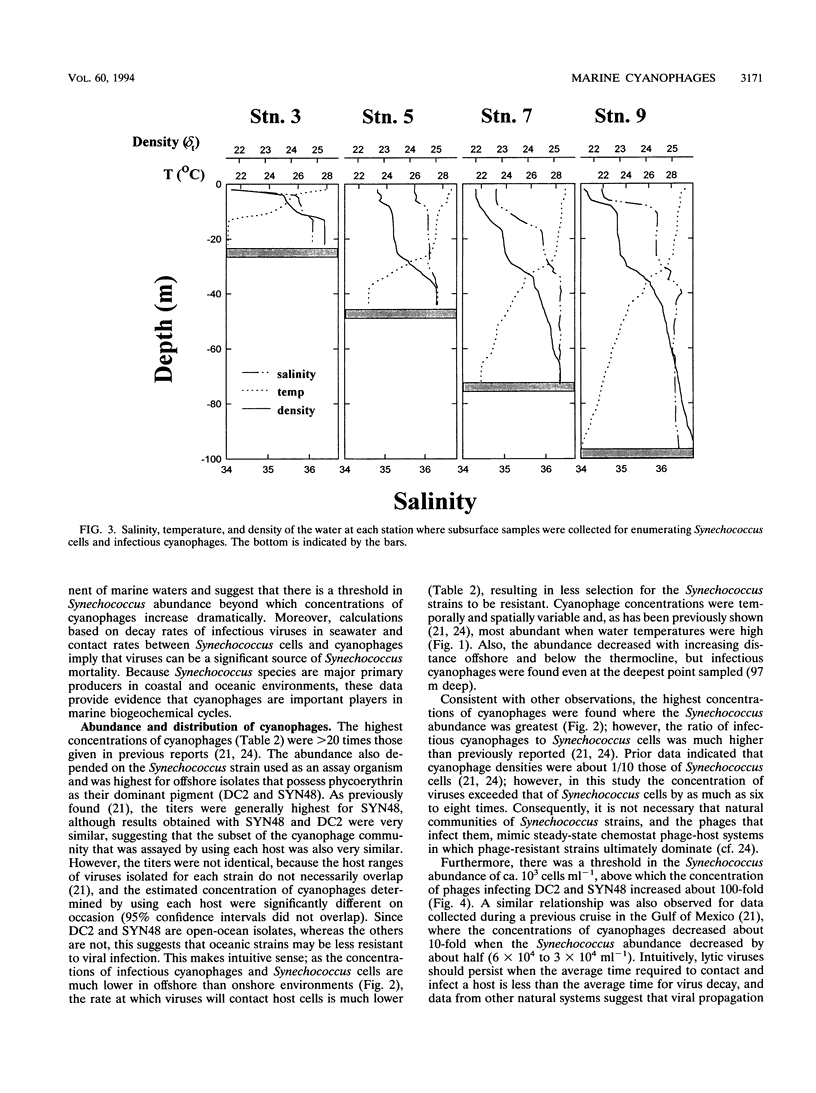
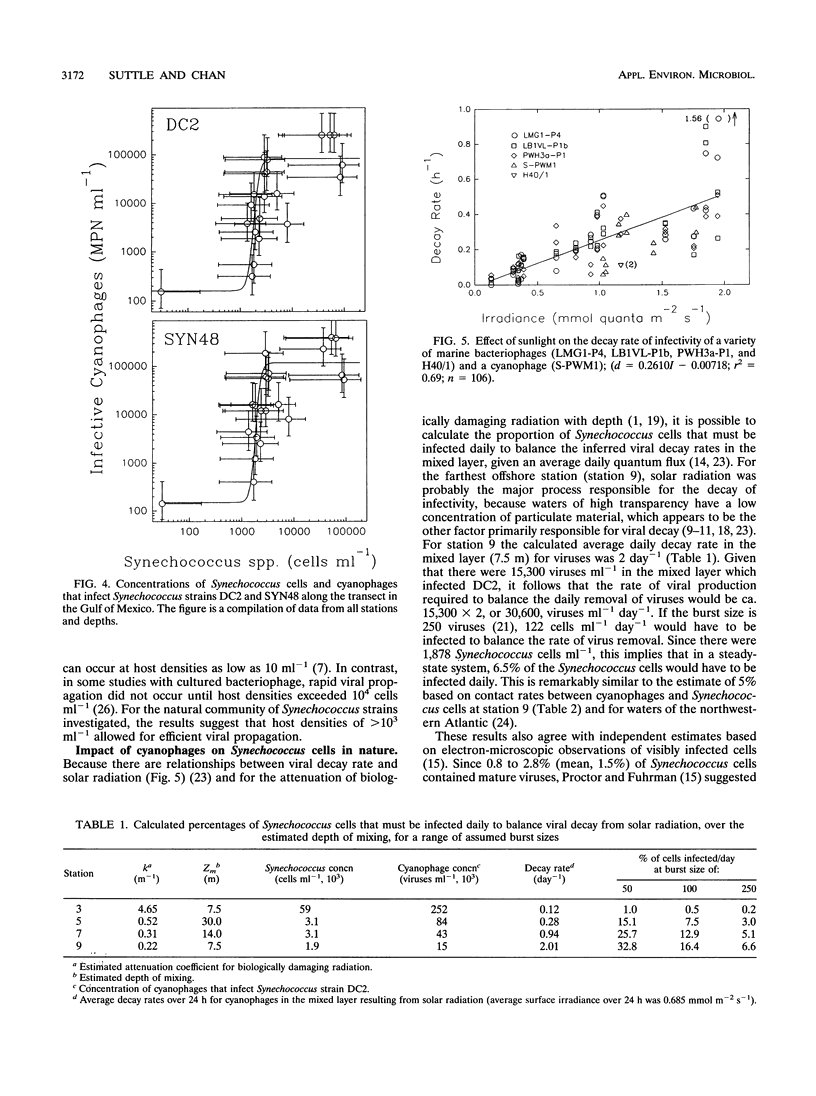
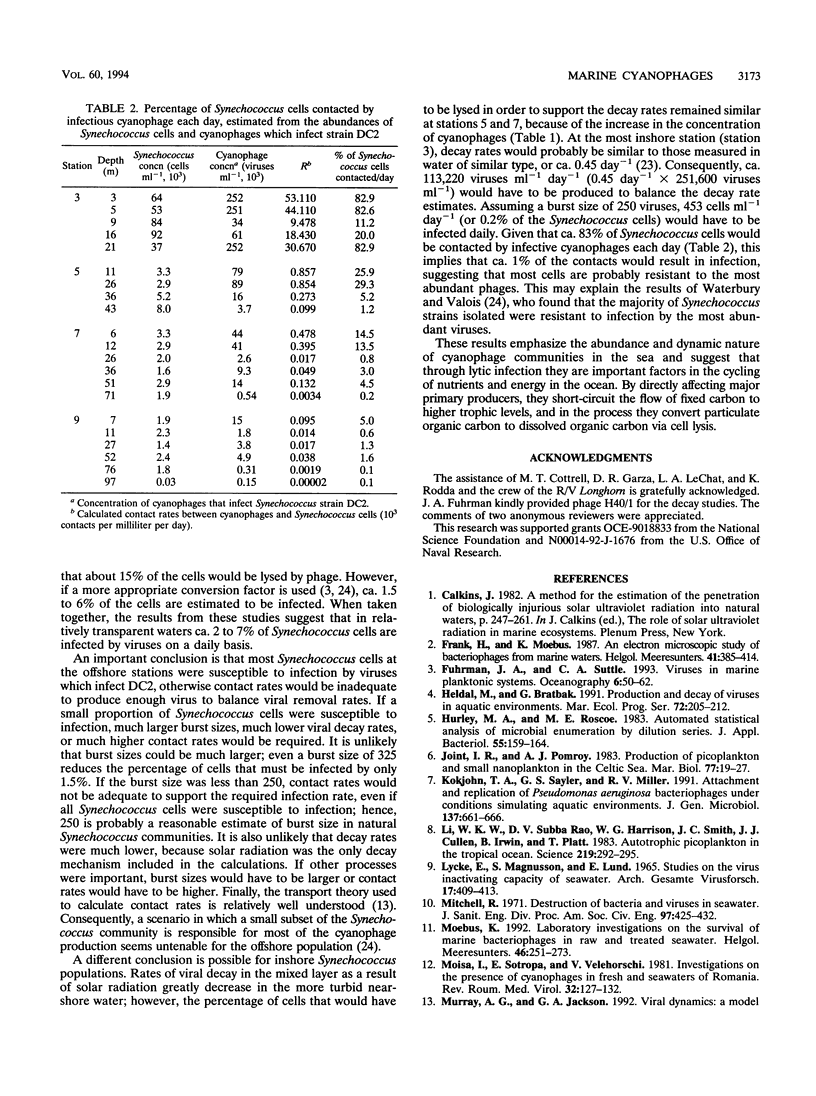
Cyanophages infecting marine Synechococcus cells were frequently very abundant and were found in every seawater sample along a transect in the western Gulf of Mexico and during a 28-month period in Aransas Pass, Tex. In Aransas Pass their abundance varied seasonally, with the lowest concentrations coincident with cooler water and lower salinity. Along the transect, viruses infecting Synechococcus strains DC2 and SYN48 ranged in concentration from a few hundred per milliliter at 97 m deep and 83 km offshore to ca. 4 × 105 ml-1 near the surface at stations within 18 km of the coast. The highest concentrations occurred at the surface, where salinity decreased from ca. 35.5 to 34 ppt and Synechococcus concentrations were greatest. Viruses infecting strains SNC1, SNC2, and 838BG were distributed in a similar manner but were much less abundant (<10 to >5 × 103 ml-1). When Synechococcus concentrations exceeded ca. 103 ml-1, cyanophage concentrations increased markedly (ca. 102 to > 105 ml-1), suggesting that a minimum host density was required for efficient viral propagation. Data on the decay rate of viral infectivity d (per day), as a function of solar irradiance I (millimoles of quanta per square meter per second), were used to develop a relationship (d = 0.2610I - 0.00718; r2 = 0.69) for conservatively estimating the destruction of infectious viruses in the mixed layer of two offshore stations. Assuming that virus production balances losses and that the burst size is 250, ca. 5 to 7% of Synechococcus cells would be infected daily by viruses. Calculations based on contact rates between Synechococcus cells and infectious viruses produce similar results (5 to 14%). Moreover, balancing estimates of viral production with contact rates for the farthest offshore station required that most Synechococcus cells be susceptible to infection, that most contacts result in infection, and that the burst size be about 324 viruses per lytic event. In contrast, in nearshore waters, where ca. 80% of Synechococcus cells would be contacted daily by infectious cyanophages, only ca. 1% of the contacts would have to result in infection to balance the estimated virus removal rates. These results indicate that cyanophages are an abundant and dynamic component of marine planktonic communities and are probably responsible for lysing a small but significant portion of the Synechococcus population on a daily basis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Li W. K., Rao D. V., Harrison W. G., Smith J. C., Cullen J. J., Irwin B., Platt T. Autotrophic picoplankton in the tropical ocean. Science. 1983 Jan 21;219(4582):292–295. doi: 10.1126/science.219.4582.292. [DOI] [PubMed] [Google Scholar]
- Lycke E., Magnusson S., Lund E. Studies on the nature of the virus inactivating capacity of sea water. Arch Gesamte Virusforsch. 1965;17(3):409–413. doi: 10.1007/BF01241195. [DOI] [PubMed] [Google Scholar]
- Moisa I., Sotropa E., Velehorschi V. Investigations on the presence of cyanophages in fresh and sea waters of Romania. Virologie. 1981 Apr-Jun;32(2):127–132. [PubMed] [Google Scholar]
- Safferman R. S., Morris M. E. Observations on the Occurrence, Distribution, and Seasonal Incidence of Blue-green Algal Viruses. Appl Microbiol. 1967 Sep;15(5):1219–1222. doi: 10.1128/am.15.5.1219-1222.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle C. A., Chen F. Mechanisms and rates of decay of marine viruses in seawater. Appl Environ Microbiol. 1992 Nov;58(11):3721–3729. doi: 10.1128/aem.58.11.3721-3729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterbury J. B., Valois F. W. Resistance to co-occurring phages enables marine synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol. 1993 Oct;59(10):3393–3399. doi: 10.1128/aem.59.10.3393-3399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins B. A., Alexander M. Minimum bacterial density for bacteriophage replication: implications for significance of bacteriophages in natural ecosystems. Appl Environ Microbiol. 1985 Jan;49(1):19–23. doi: 10.1128/aem.49.1.19-23.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. H., Joint I. R., Carr N. G., Mann N. H. Isolation and Molecular Characterization of Five Marine Cyanophages Propagated on Synechococcus sp. Strain WH7803. Appl Environ Microbiol. 1993 Nov;59(11):3736–3743. doi: 10.1128/aem.59.11.3736-3743.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]



