Abstract
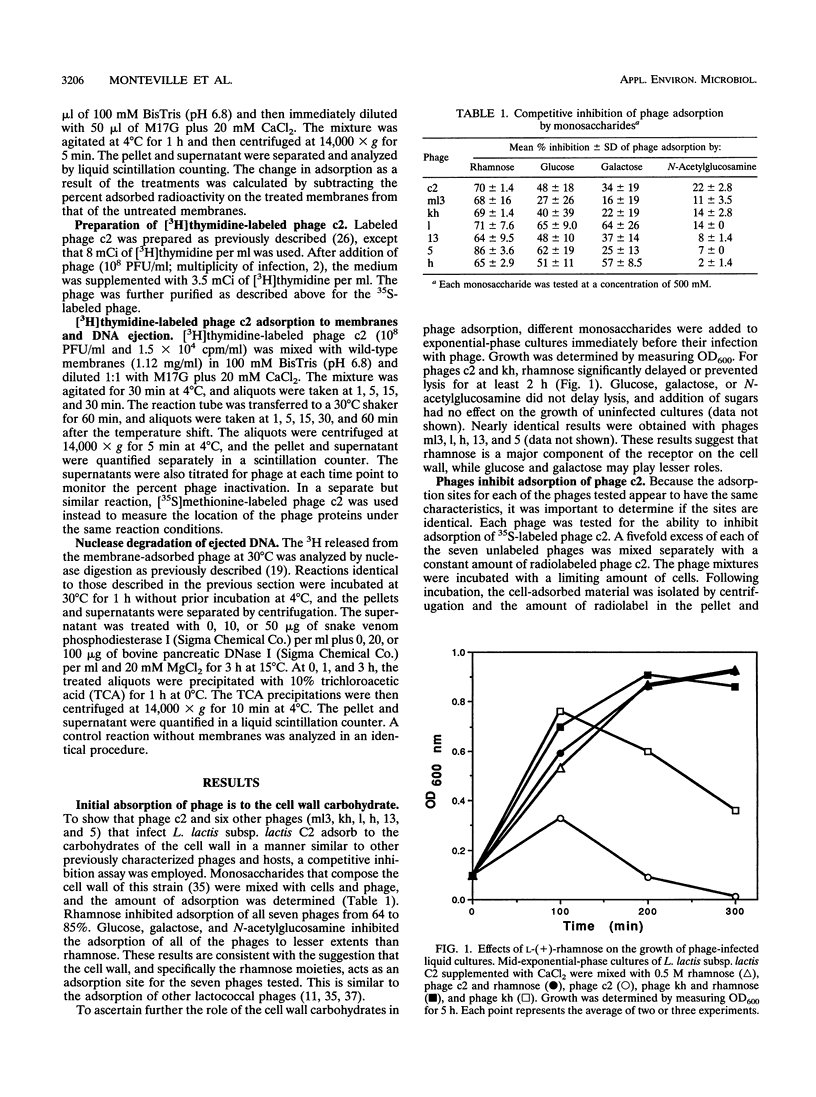
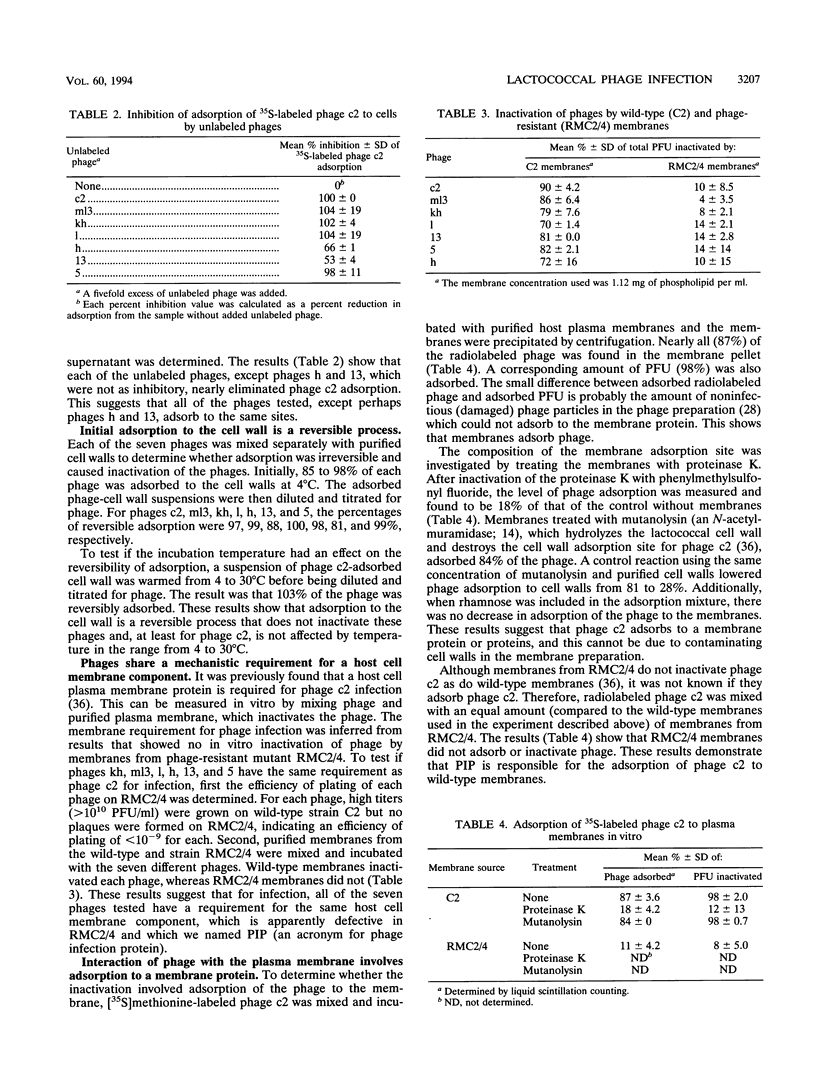
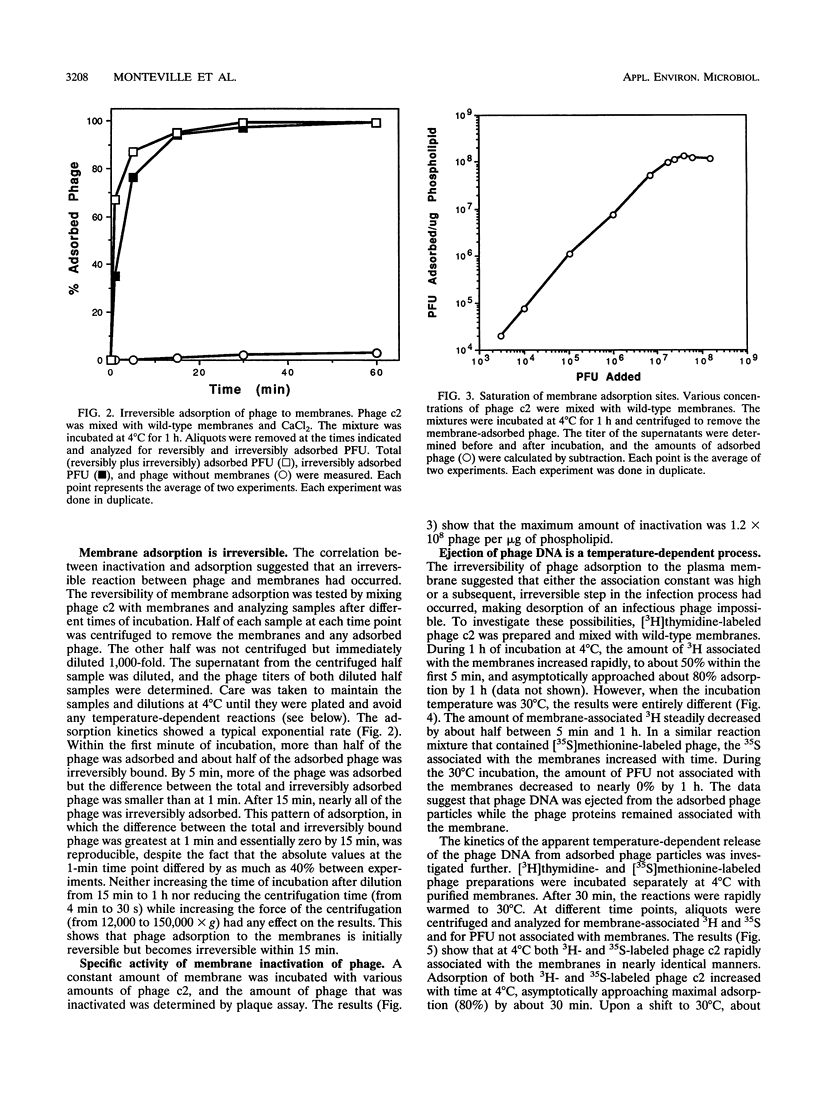
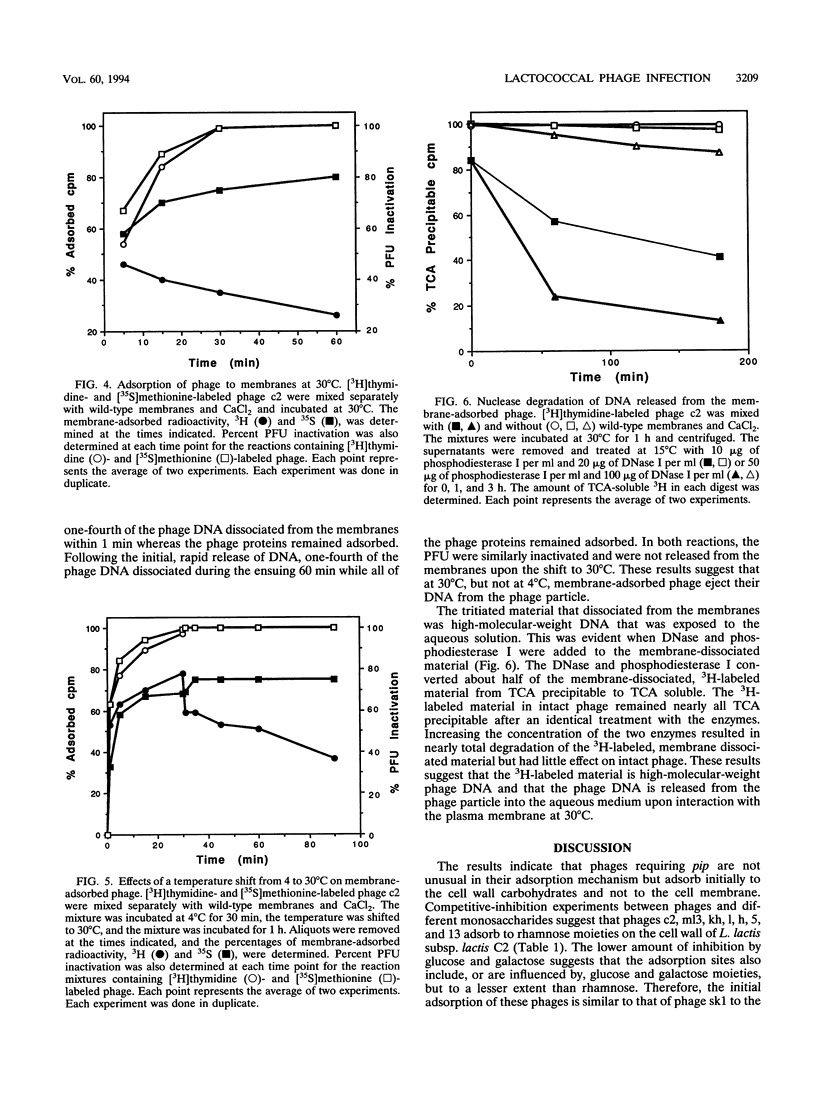
The mechanism of the initial steps of bacteriophage infection in Lactococcus lactis subsp. lactis C2 was investigated by using phages c2, ml3, kh, l, h, 5, and 13. All seven phages adsorbed to the same sites on the host cell wall that are composed, in part, of rhamnose. This was suggested by rhamnose inhibition of phage adsorption to cells, competition between phage c2 and the other phages for adsorption to cells, and rhamnose inhibition of lysis of phage-inoculated cultures. The adsorption to the cell wall was found to be reversible upon dilution of the cell wall-adsorbed phage. In a reaction step that apparently follows adsorption to the cell wall, all seven phages adsorbed to a host membrane protein named PIP. This was indicated by the inability of all seven phages to infect a strain selected for resistance to phage c2 and known to have a defective PIP protein. All seven phages were inactivated in vitro by membranes from wild-type cells but not by membranes from the PIP-defective, phage c2-resistant strain. The mechanism of membrane inactivation was an irreversible adsorption of the phage to PIP, as indicated by adsorption of [35S] methionine-labeled phage c2 to purified membranes from phage-sensitive cells but not to membranes from the resistant strain, elimination of adsorption by pretreatment of the membranes with proteinase K, and lack of dissociation of 35S from the membranes upon dilution. Following membrane adsorption, ejection of phage DNA occurred rapidly at 30°C but not at 4°C. These results suggest that many lactococcal phages adsorb initially to the cell wall and subsequently to host cell membrane protein PIP, which leads to ejection of the phage genome.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- American Dairy Science Association. American Society of Animal Science. Annual meeting. July 11-15, 1994, Minneapolis, Minnesota. Abstracts. J Dairy Sci. 1994;77 (Suppl 1):1–394. [PubMed] [Google Scholar]
- Chopin A., Chopin M. C., Moillo-Batt A., Langella P. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984 May;11(3):260–263. doi: 10.1016/0147-619x(84)90033-7. [DOI] [PubMed] [Google Scholar]
- Froseth B. R., Harlander S. K., McKay L. L. Plasmid-mediated reduced phage sensitivity in Streptococcus lactis KR5. J Dairy Sci. 1988 Feb;71(2):275–284. doi: 10.3168/jds.S0022-0302(88)79555-7. [DOI] [PubMed] [Google Scholar]
- Furukawa H., Mizushima S. Roles of cell surface components of Escherichia coli K-12 in bacteriophage T4 infection: interaction of tail core with phospholipids. J Bacteriol. 1982 May;150(2):916–924. doi: 10.1128/jb.150.2.916-924.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B. L., Ivey R. G., Trempy J. E., Hettinger-Smith B. Cloning of a chromosomal gene required for phage infection of Lactococcus lactis subsp. lactis C2. J Bacteriol. 1993 Sep;175(17):5510–5519. doi: 10.1128/jb.175.17.5510-5519.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal P. K., Crow V. L. Characterization of Loosely Associated Material from the Cell Surface of Lactococcus lactis subsp. cremoris E8 and Its Phage-Resistant Variant Strain 398. Appl Environ Microbiol. 1993 Oct;59(10):3177–3182. doi: 10.1128/aem.59.10.3177-3182.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. L., Sanozky-Dawes R. B., Klaenhammer T. R. Restriction and modification activities from Streptococcus lactis ME2 are encoded by a self-transmissible plasmid, pTN20, that forms cointegrates during mobilization of lactose-fermenting ability. J Bacteriol. 1988 Aug;170(8):3435–3442. doi: 10.1128/jb.170.8.3435-3442.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C., Romero D. A., McKenney D. S., Finer K. R., Klaenhammer T. R. Localization, cloning, and expression of genetic determinants for bacteriophage resistance (Hsp) from the conjugative plasmid pTR2030. Appl Environ Microbiol. 1989 Jul;55(7):1684–1689. doi: 10.1128/aem.55.7.1684-1689.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona N. L., Selvidge L. Mechanism of adsorption and eclipse of bacteriophage phi X174. II. Attachment and eclipse with isolated Escherichia coli cell wall lipopolysaccharide. J Virol. 1973 May;11(5):775–782. doi: 10.1128/jvi.11.5.775-782.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh B. P., Pettingill G. Adsorption of Bacteriophage eb7 on Streptococcus cremoris EB7. Appl Environ Microbiol. 1983 Jun;45(6):1946–1948. doi: 10.1128/aem.45.6.1946-1948.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., Sanozky R. B. Conjugal transfer from Streptococcus lactis ME2 of plasmids encoding phage resistance, nisin resistance and lactose-fermenting ability: evidence for a high-frequency conjugative plasmid responsible for abortive infection of virulent bacteriophage. J Gen Microbiol. 1985 Jun;131(6):1531–1541. doi: 10.1099/00221287-131-6-1531. [DOI] [PubMed] [Google Scholar]
- Labedan B., Goldberg E. B. Requirement for membrane potential in injection of phage T4 DNA. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4669–4673. doi: 10.1073/pnas.76.9.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mackay D. J., Bode V. C. Binding to isolated phage receptors and lambda DNA release in vitro. Virology. 1976 Jul 1;72(1):167–181. doi: 10.1016/0042-6822(76)90321-4. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Conjugative 40-megadalton plasmid in Streptococcus lactis subsp. diacetylactis DRC3 is associated with resistance to nisin and bacteriophage. Appl Environ Microbiol. 1984 Jan;47(1):68–74. doi: 10.1128/aem.47.1.68-74.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold J. E., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XXXIV. Kinetic of the attachment and eclipse steps of the infection. J Virol. 1970 Apr;5(4):427–431. doi: 10.1128/jvi.5.4.427-431.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oram J. D. Isolation and properties of a phage receptor substance from the plasma membrane of Streptococcus lactis ML 3. J Gen Virol. 1971 Oct;13(1):59–71. doi: 10.1099/0022-1317-13-1-59. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The adsorption of phage to group N streptococci. The specificity of adsorption and the location of phage receptor substances in cell-wall and plasma-membrane fractions. J Gen Virol. 1968 Jul;3(1):103–119. doi: 10.1099/0022-1317-3-1-103. [DOI] [PubMed] [Google Scholar]
- Powell I. B., Tulloch D. L., Hillier A. J., Davidson B. E. Phage DNA synthesis and host DNA degradation in the life cycle of Lactococcus lactis bacteriophage c6A. J Gen Microbiol. 1992 May;138(5):945–950. doi: 10.1099/00221287-138-5-945. [DOI] [PubMed] [Google Scholar]
- Randall-Hazelbauer L., Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973 Dec;116(3):1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa M., Scandella D. Multiple steps during the interaction between coliphage lambda and its receptor protein in vitro. Virology. 1976 Jul 1;72(1):182–194. doi: 10.1016/0042-6822(76)90322-6. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Characterization of Phage-Sensitive Mutants from a Phage-Insensitive Strain of Streptococcus lactis: Evidence for a Plasmid Determinant that Prevents Phage Adsorption. Appl Environ Microbiol. 1983 Nov;46(5):1125–1133. doi: 10.1128/aem.46.5.1125-1133.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Klaenhammer T. R. Evidence for Plasmid Linkage of Restriction and Modification in Streptococcus cremoris KH. Appl Environ Microbiol. 1981 Dec;42(6):944–950. doi: 10.1128/aem.42.6.944-950.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijtsma L., Jansen N., Hazeleger W. C., Wouters J. T., Hellingwerf K. J. Cell Surface Characteristics of Bacteriophage-Resistant Lactococcus lactis subsp. cremoris SK110 and Its Bacteriophage-Sensitive Variant SK112. Appl Environ Microbiol. 1990 Oct;56(10):3230–3233. doi: 10.1128/aem.56.10.3230-3233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijtsma L., Sterkenburg A., Wouters J. T. Properties of the Cell Walls of Lactococcus lactis subsp. cremoris SK110 and SK112 and Their Relation to Bacteriophage Resistance. Appl Environ Microbiol. 1988 Nov;54(11):2808–2811. doi: 10.1128/aem.54.11.2808-2811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyasevi R., Sandine W. E., Geller B. L. A membrane protein is required for bacteriophage c2 infection of Lactococcus lactis subsp. lactis C2. J Bacteriol. 1991 Oct;173(19):6095–6100. doi: 10.1128/jb.173.19.6095-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyasevi R., Sandine W. E., Geller B. L. The bacteriophage kh receptor of Lactococcus lactis subsp. cremoris KH is the rhamnose of the extracellular wall polysaccharide. Appl Environ Microbiol. 1990 Jun;56(6):1882–1889. doi: 10.1128/aem.56.6.1882-1889.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Nakashima Y., Kamiya S. Effects of some L-rhamnosyl derivatives on the adsorption of phage PL-1 to the host Lactobacillus casei. Biosci Biotechnol Biochem. 1992 Feb;56(2):346–346. doi: 10.1271/bbb.56.346. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Takesue S., Ishibashi K. Adenosine triphosphate content in Lactobacillus casei and the blender-resistant phage-cell complex-forming ability of cells on infection with PL-1 phage. J Gen Virol. 1979 Jan;42(1):27–36. doi: 10.1099/0022-1317-42-1-27. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Takesue S. The requirement for calcium in infection with Lactobacillus phage. J Gen Virol. 1972 Oct;17(1):19–30. doi: 10.1099/0022-1317-17-1-19. [DOI] [PubMed] [Google Scholar]
- Wilson J. H., Luftig R. B., Wood W. B. Interaction of bacteriophage T4 tail fiber components with a lipopolysaccharide fraction from Escherichia coli. J Mol Biol. 1970 Jul 28;51(2):423–434. doi: 10.1016/0022-2836(70)90152-x. [DOI] [PubMed] [Google Scholar]
- Yokokura T. Phage receptor material in Lactobacillus casei cell wall. I. Effect of L-rhamnose on phage adsorption to the cell wall. Jpn J Microbiol. 1971 Sep;15(5):457–463. doi: 10.1111/j.1348-0421.1971.tb00604.x. [DOI] [PubMed] [Google Scholar]



