Abstract
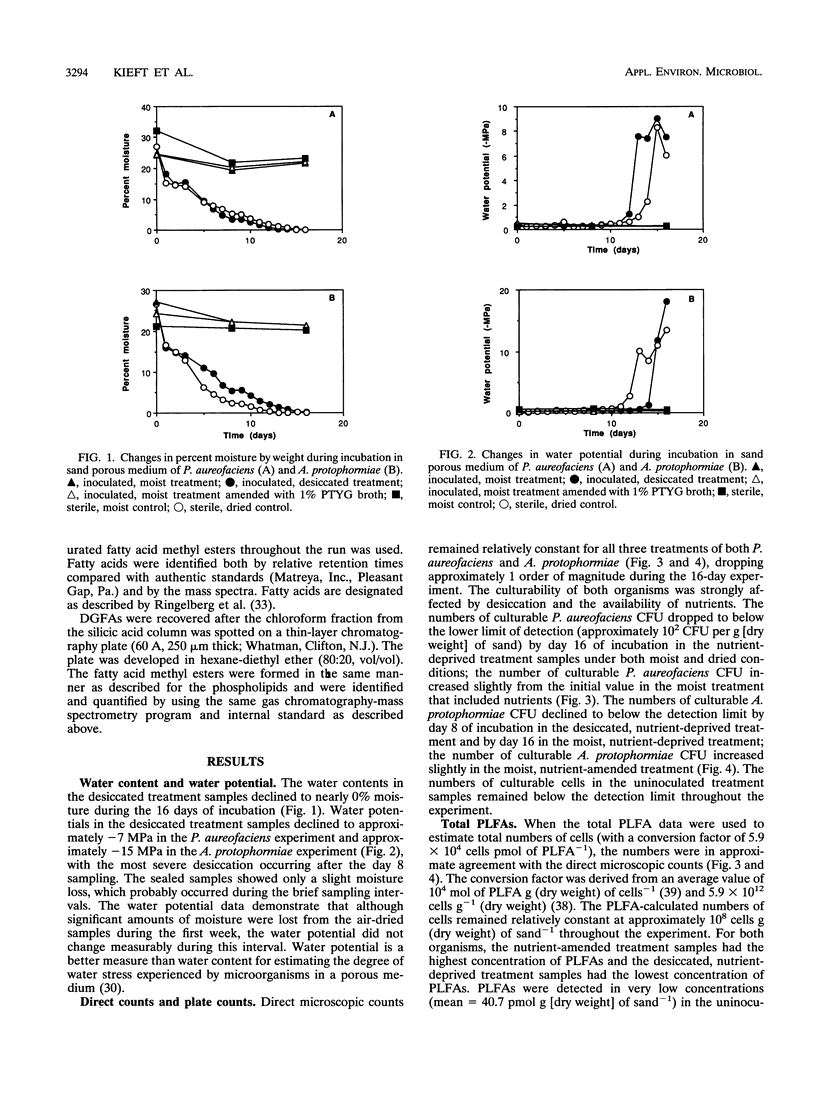
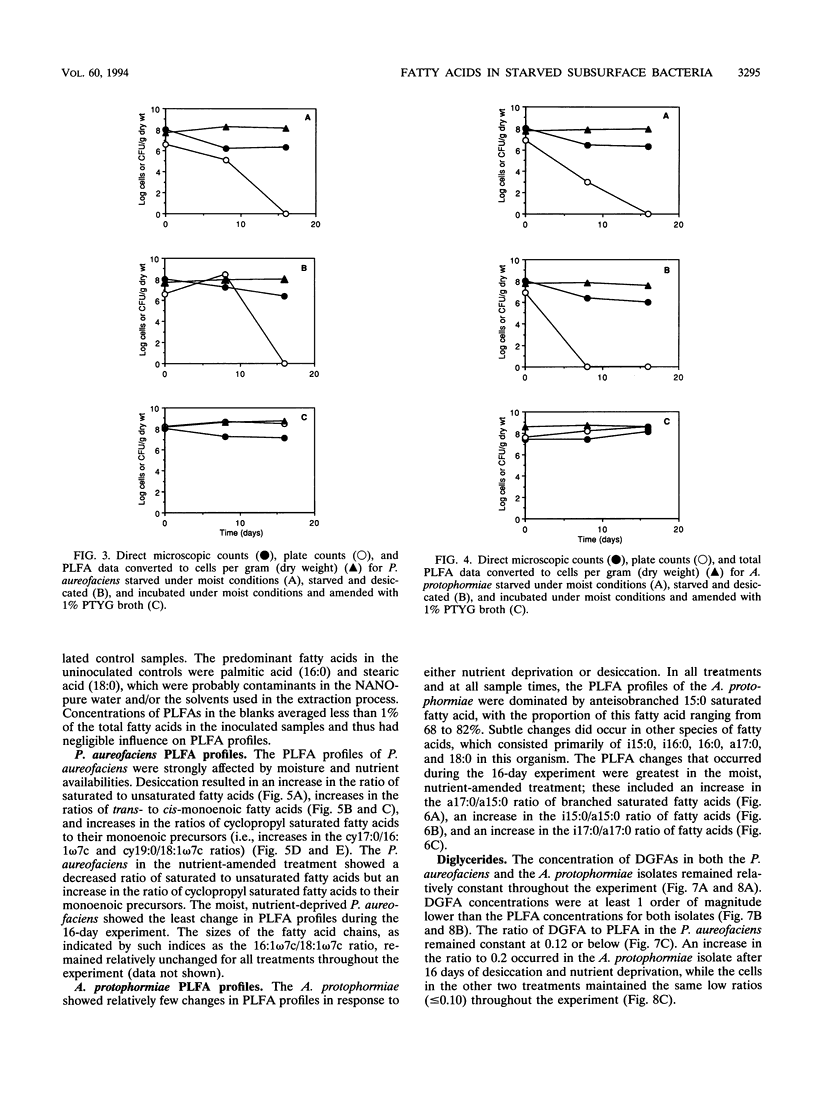
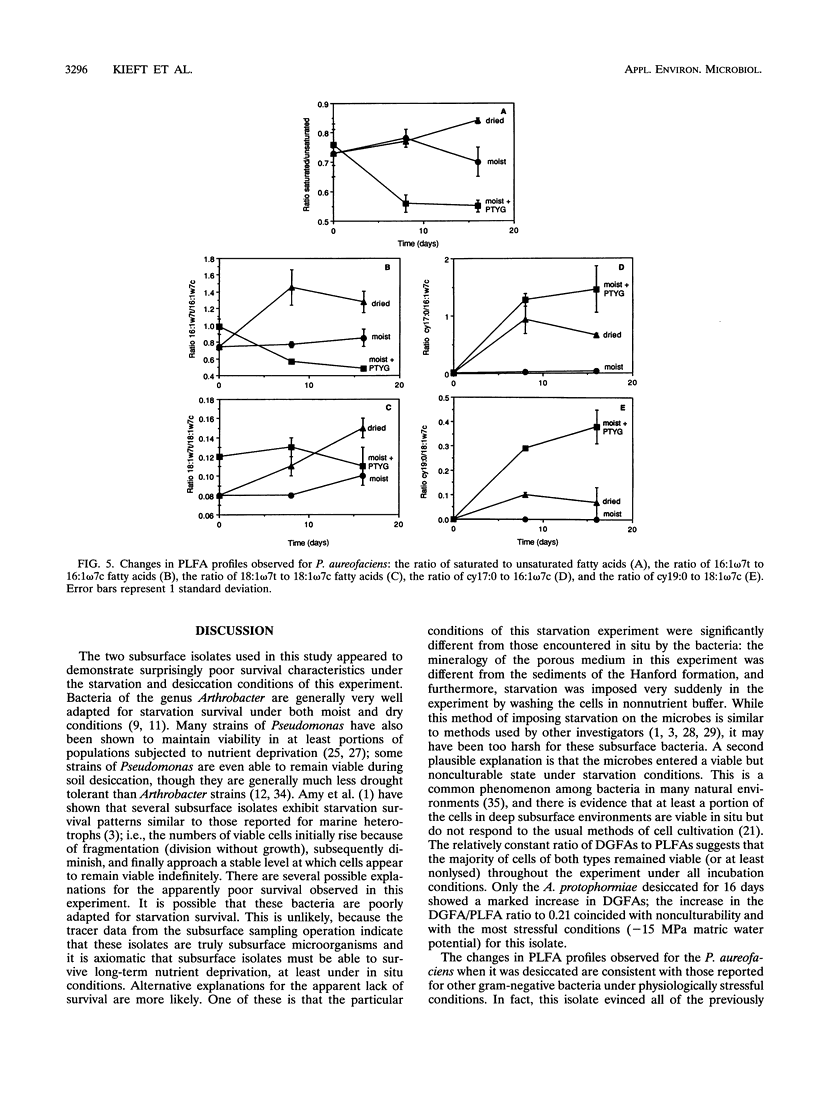
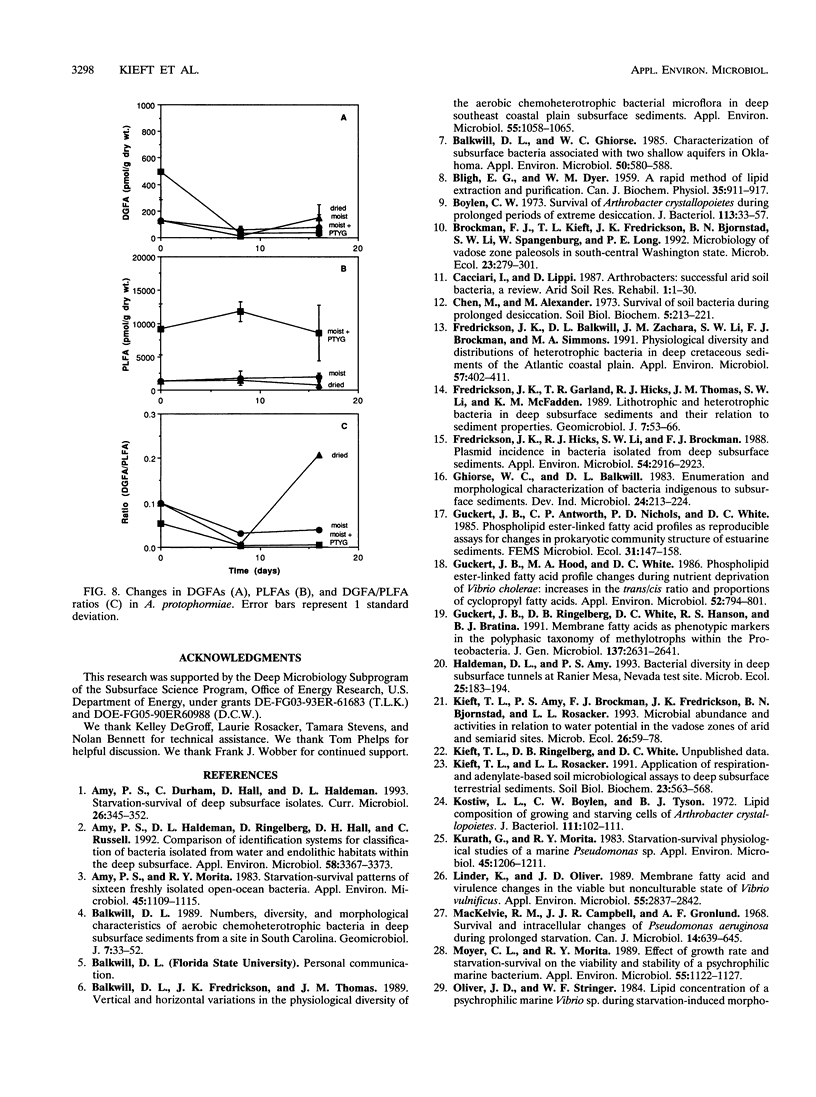
Ester-linked phospholipid fatty acid (PLFA) profiles of a Pseudomonas aureofaciens strain and an Arthrobacter protophormiae strain, each isolated from a subsurface sediment, were quantified in a starvation experiment in a silica sand porous medium under moist and dry conditions. Washed cells were added to sand microcosms and maintained under saturated conditions or subjected to desiccation by slow drying over a period of 16 days to final water potentials of approximately - 7.5 MPa for the P. aureofaciens and - 15 MPa for the A. protophormiae. In a third treatment, cells were added to saturated microcosms along with organic nutrients and maintained under saturated conditions. The numbers of culturable cells of both bacterial strains declined to below detection level within 16 days in both the moist and dried nutrient-deprived conditions, while direct counts and total PLFAs remained relatively constant. Both strains of bacteria maintained culturability in the nutrient-amended microcosms. The dried P. aureofaciens cells showed changes in PLFA profiles that are typically associated with stressed gram-negative cells, i.e., increased ratios of saturated to unsaturated fatty acids, increased ratios of trans- to cis-monoenoic fatty acids, and increased ratios of cyclopropyl fatty acids to their monoenoic precursors. P. aureofaciens starved under moist conditions showed few changes in PLFA profiles during the 16-day incubation, whereas cells incubated in the presence of nutrients showed decreases in the ratios of both saturated fatty acids to unsaturated fatty acids and cyclopropyl fatty acids to their monoenoic precursors. The PLFA profiles of A. protophormiae changed very little in response to either nutrient deprivation or desiccation. Diglyceride fatty acids, which have been proposed to be indicators of dead or lysed cells, remained relatively constant throughout the experiment. Only the A. protophormiae desiccated for 16 days showed an increase in the ratio of diglyceride fatty acids to PLFAs. The results of this laboratory experiment can be useful for interpreting PLFA profiles of subsurface communities of microorganisms for the purpose of determining their physiological status.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Haldeman D. L., Ringelberg D., Hall D. H., Russell C. Comparison of Identification Systems for Classification of Bacteria Isolated from Water and Endolithic Habitats within the Deep Subsurface. Appl Environ Microbiol. 1992 Oct;58(10):3367–3373. doi: 10.1128/aem.58.10.3367-3373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy P. S., Morita R. Y. Starvation-survival patterns of sixteen freshly isolated open-ocean bacteria. Appl Environ Microbiol. 1983 Mar;45(3):1109–1115. doi: 10.1128/aem.45.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Balkwill D. L., Fredrickson J. K., Thomas J. M. Vertical and horizontal variations in the physiological diversity of the aerobic chemoheterotrophic bacterial microflora in deep southeast coastal plain subsurface sediments. Appl Environ Microbiol. 1989 May;55(5):1058–1065. doi: 10.1128/aem.55.5.1058-1065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill D. L., Ghiorse W. C. Characterization of subsurface bacteria associated with two shallow aquifers in oklahoma. Appl Environ Microbiol. 1985 Sep;50(3):580–588. doi: 10.1128/aem.50.3.580-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylen C. W. Survival of Arthrobacter crystallopoietes during prolonged periods of extreme desiccation. J Bacteriol. 1973 Jan;113(1):33–37. doi: 10.1128/jb.113.1.33-37.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Francisco A., Hall A. J., Schellenberg J. R., Greenwood A. M., Greenwood B. M. The pattern of infant and childhood mortality in Upper River Division, The Gambia. Ann Trop Paediatr. 1993;13(4):345–352. doi: 10.1080/02724936.1993.11747669. [DOI] [PubMed] [Google Scholar]
- Fredrickson J. K., Balkwill D. L., Zachara J. M., Li S. M., Brockman F. J., Simmons M. A. Physiological diversity and distributions of heterotrophic bacteria in deep cretaceous sediments of the atlantic coastal plain. Appl Environ Microbiol. 1991 Feb;57(2):402–411. doi: 10.1128/aem.57.2.402-411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson J. K., Hicks R. J., Li S. W., Brockman F. J. Plasmid incidence in bacteria from deep subsurface sediments. Appl Environ Microbiol. 1988 Dec;54(12):2916–2923. doi: 10.1128/aem.54.12.2916-2923.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckert J. B., Hood M. A., White D. C. Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae: increases in the trans/cis ratio and proportions of cyclopropyl fatty acids. Appl Environ Microbiol. 1986 Oct;52(4):794–801. doi: 10.1128/aem.52.4.794-801.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckert J. B., Ringelberg D. B., White D. C., Hanson R. S., Bratina B. J. Membrane fatty acids as phenotypic markers in the polyphasic taxonomy of methylotrophs within the Proteobacteria. J Gen Microbiol. 1991 Nov;137(11):2631–2641. doi: 10.1099/00221287-137-11-2631. [DOI] [PubMed] [Google Scholar]
- Kostiw L. L., Boylen C. W., Tyson B. J. Lipid composition of growing and starving cells of Arthrobacter crystallopoietes. J Bacteriol. 1972 Jul;111(1):103–111. doi: 10.1128/jb.111.1.103-111.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurath G., Morita R. Y. Starvation-Survival Physiological Studies of a Marine Pseudomonas sp. Appl Environ Microbiol. 1983 Apr;45(4):1206–1211. doi: 10.1128/aem.45.4.1206-1211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder K., Oliver J. D. Membrane fatty acid and virulence changes in the viable but nonculturable state of Vibrio vulnificus. Appl Environ Microbiol. 1989 Nov;55(11):2837–2842. doi: 10.1128/aem.55.11.2837-2842.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKelvie R. M., Campbell J. J., Gronlund A. F. Survival and intracellular changes of Pseudomonas aeruginosa during prolonged starvation. Can J Microbiol. 1968 Jun;14(6):639–645. doi: 10.1139/m68-107. [DOI] [PubMed] [Google Scholar]
- Moyer C. L., Morita R. Y. Effect of growth rate and starvation-survival on the viability and stability of a psychrophilic marine bacterium. Appl Environ Microbiol. 1989 May;55(5):1122–1127. doi: 10.1128/aem.55.5.1122-1127.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Stringer W. F. Lipid Composition of a Psychrophilic Marine Vibrio sp. During Starvation-Induced Morphogenesis. Appl Environ Microbiol. 1984 Mar;47(3):461–466. doi: 10.1128/aem.47.3.461-466.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts M., Olie J. J., Nickels J. S., Parsons J., White D. C. Variation in Phospholipid Ester-Linked Fatty Acids and Carotenoids of Desiccated Nostoc commune (Cyanobacteria) from Different Geographic Locations. Appl Environ Microbiol. 1987 Jan;53(1):4–9. doi: 10.1128/aem.53.1.4-9.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Oliver J. D. Starvation Response of the Marine Barophile CNPT-3. Appl Environ Microbiol. 1992 Aug;58(8):2432–2437. doi: 10.1128/aem.58.8.2432-2437.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. B., Salonius P. O., Chase F. E. A note on the differential response of arthrobacter spp. and pseudomonas spp. to drying in soil. Can J Microbiol. 1965 Aug;11(4):746–748. doi: 10.1139/m65-100. [DOI] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



