Abstract
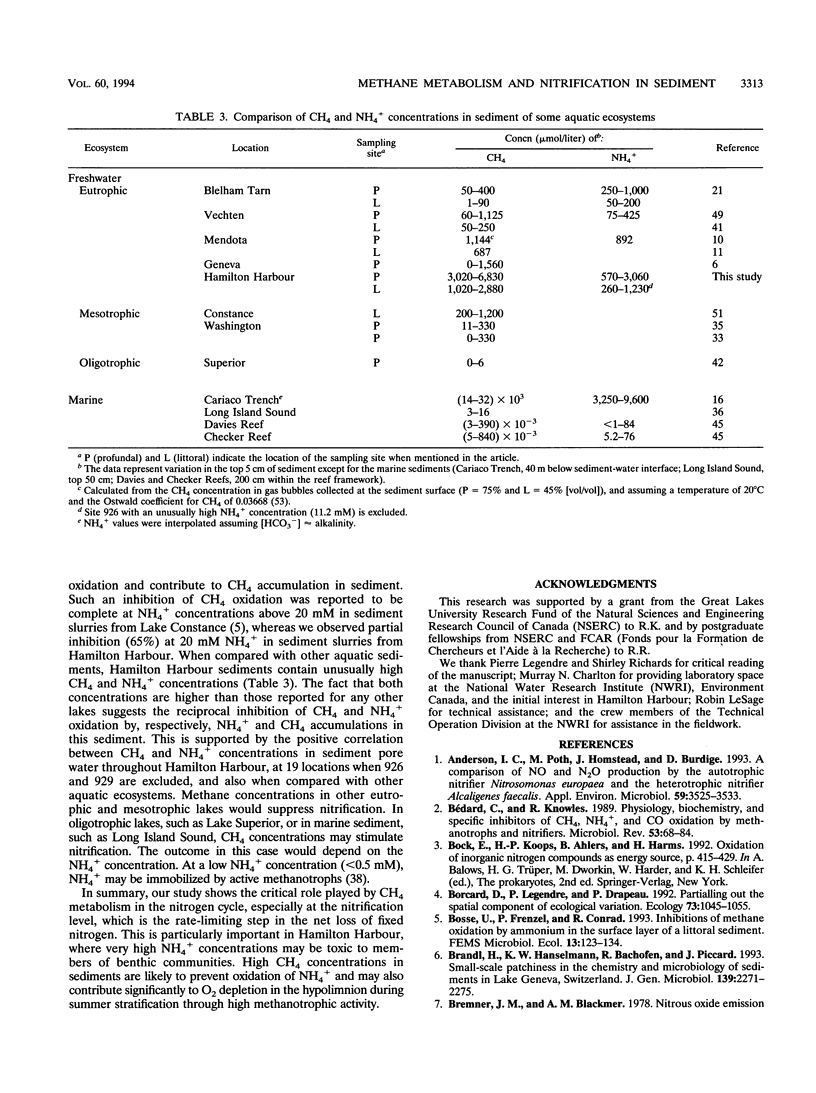
We report the effect of CH4 and of CH4 oxidation on nitrification in freshwater sediment from Hamilton Harbour, Ontario, Canada, a highly polluted ecosystem. Aerobic slurry experiments showed a high potential for aerobic N2O production in some sites. It was suppressed by C2H2, correlated to NO3- production, and stimulated by NH4+ concentration, supporting the hypothesis of a nitrification-dependent source for this N2O production. Diluted sediment slurries supplemented with CH4 (1 to 24 μM) showed earlier and enhanced nitrification and N2O production compared with unsupplemented slurries (≤1 μM CH4). This suggests that nitrification by methanotrophs may be significant in freshwater sediment under certain conditions. Suppression of nitrification was observed at CH4 concentrations of 84 μM and greater, possibly through competition for O2 between methanotrophs and NH4+ -oxidizing bacteria and/or competition for mineral N between these two groups of organisms. In Hamilton Harbour sediment, the very high CH4 concentrations (1.02 to 6.83 mM) which exist would probably suppress nitrification and favor NH4+ accumulation in the pore water. Indeed, NH4+ concentrations in Hamilton Harbour sediment are higher than those found in other lakes. We conclude that the impact of CH4 metabolism on N cycling processes in freshwater ecosystems should be given more attention.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Poth M., Homstead J., Burdige D. A comparison of NO and N2O production by the autotrophic nitrifier Nitrosomonas europaea and the heterotrophic nitrifier Alcaligenes faecalis. Appl Environ Microbiol. 1993 Nov;59(11):3525–3533. doi: 10.1128/aem.59.11.3525-3533.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. M., Blackmer A. M. Nitrous oxide: emission from soils during nitrification of fertilizer nitrogen. Science. 1978 Jan 20;199(4326):295–296. doi: 10.1126/science.199.4326.295. [DOI] [PubMed] [Google Scholar]
- Bédard C., Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989 Mar;53(1):68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goreau T. J., Kaplan W. A., Wofsy S. C., McElroy M. B., Valois F. W., Watson S. W. Production of NO(2) and N(2)O by Nitrifying Bacteria at Reduced Concentrations of Oxygen. Appl Environ Microbiol. 1980 Sep;40(3):526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTON W. E., ZOBELL C. E. Production of nitrite from ammonia by methane oxidizing bacteria. J Bacteriol. 1953 Feb;65(2):216–219. doi: 10.1128/jb.65.2.216-219.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke R. A., Giesy J. P., Kreis R. G., Jr Sediment pore water toxicity identification in the lower Fox River and Green Bay, Wisconsin, using the Microtox assay. Ecotoxicol Environ Saf. 1992 Jun;23(3):343–354. doi: 10.1016/0147-6513(92)90083-f. [DOI] [PubMed] [Google Scholar]
- Keener W. K., Arp D. J. Kinetic Studies of Ammonia Monooxygenase Inhibition in Nitrosomonas europaea by Hydrocarbons and Halogenated Hydrocarbons in an Optimized Whole-Cell Assay. Appl Environ Microbiol. 1993 Aug;59(8):2501–2510. doi: 10.1128/aem.59.8.2501-2510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith K. M., Alexander V. Sediment nitrification, denitrification, and nitrous oxide production in a deep arctic lake. Appl Environ Microbiol. 1983 Nov;46(5):1084–1092. doi: 10.1128/aem.46.5.1084-1092.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. Denitrification. Microbiol Rev. 1982 Mar;46(1):43–70. doi: 10.1128/mr.46.1.43-70.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidstrom M. E., Somers L. Seasonal study of methane oxidation in lake washington. Appl Environ Microbiol. 1984 Jun;47(6):1255–1260. doi: 10.1128/aem.47.6.1255-1260.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osen-Sand A., Catsicas M., Staple J. K., Jones K. A., Ayala G., Knowles J., Grenningloh G., Catsicas S. Inhibition of axonal growth by SNAP-25 antisense oligonucleotides in vitro and in vivo. Nature. 1993 Jul 29;364(6436):445–448. doi: 10.1038/364445a0. [DOI] [PubMed] [Google Scholar]
- Schimel J. P., Firestone M. K., Killham K. S. Identification of heterotrophic nitrification in a sierran forest soil. Appl Environ Microbiol. 1984 Oct;48(4):802–806. doi: 10.1128/aem.48.4.802-806.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari T. Nitrite and nitrous oxide production by Methylosinus trichosporium. Can J Microbiol. 1985 Feb;31(2):139–144. doi: 10.1139/m85-027. [DOI] [PubMed] [Google Scholar]



