Abstract
Crude bean root extracts of Phaseolus vulgaris were tested for inhibition of the growth of several polysaccharide mutants of Rhizobium etli biovar phaseoli CE3. Mutants deficient only in exopolysaccharide and some mutants deficient only in the O-antigen of the lipopolysaccharide were no more sensitive than the wild-type strain to the extracts, whereas mutants defective in both lipopolysaccharide and exopolysaccharide were much more sensitive. The inhibitory activity was found at much higher levels in roots and nodules than in stems or leaves. Inoculation with either wild-type or polysaccharide-deficient R. etli did not appear to affect the level of activity. Sequential extractions of the crude root material with petroleum ether, ethyl acetate, methanol, and water partitioned inhibitory activity into each solvent except methanol. The major inhibitors in the petroleum ether and ethyl acetate extracts were purified by C18 high-performance liquid chromatography. These compounds all migrated very similarly in both liquid and thin-layer chromatography but were distinguished by their mass spectra. Absorbance spectra and fluorescence properties suggested that they were coumestans, one of which had the mass spectrum and nuclear magnetic resonances of coumestrol. These results are discussed with regard to the hypothesis that one role of rhizobial polysaccharides is to protect against plant toxins encountered during nodule development.
Full text
PDF

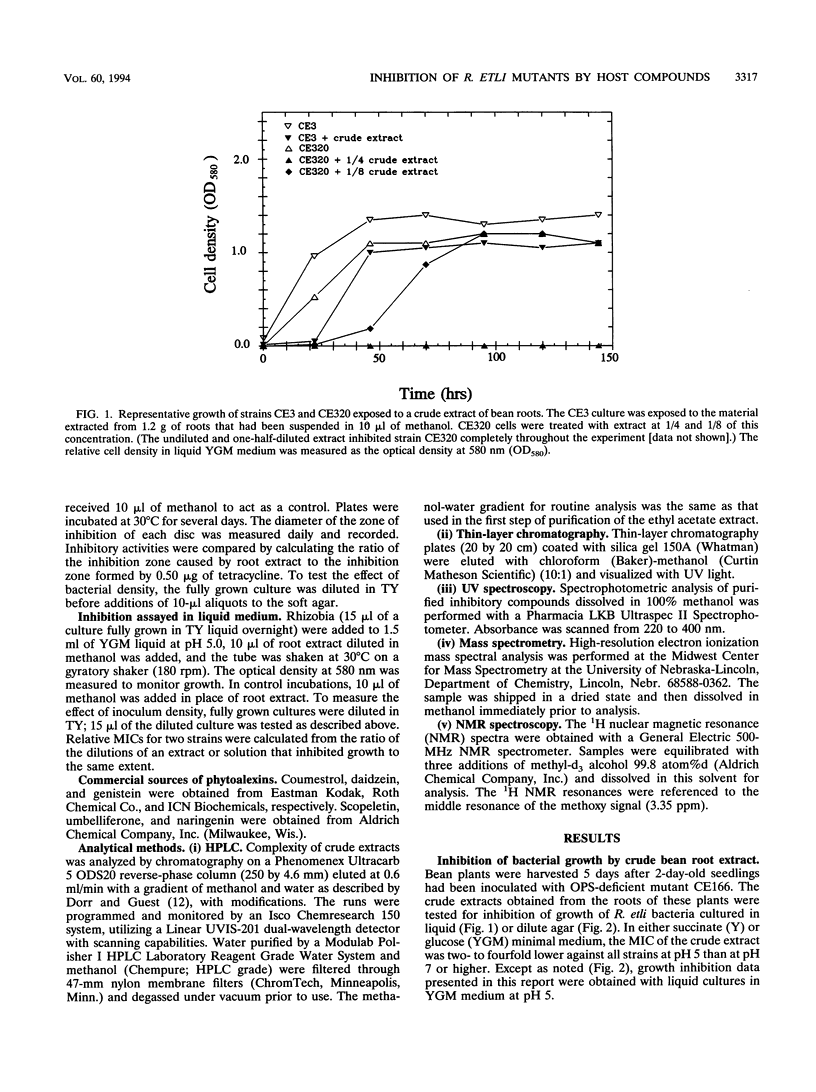
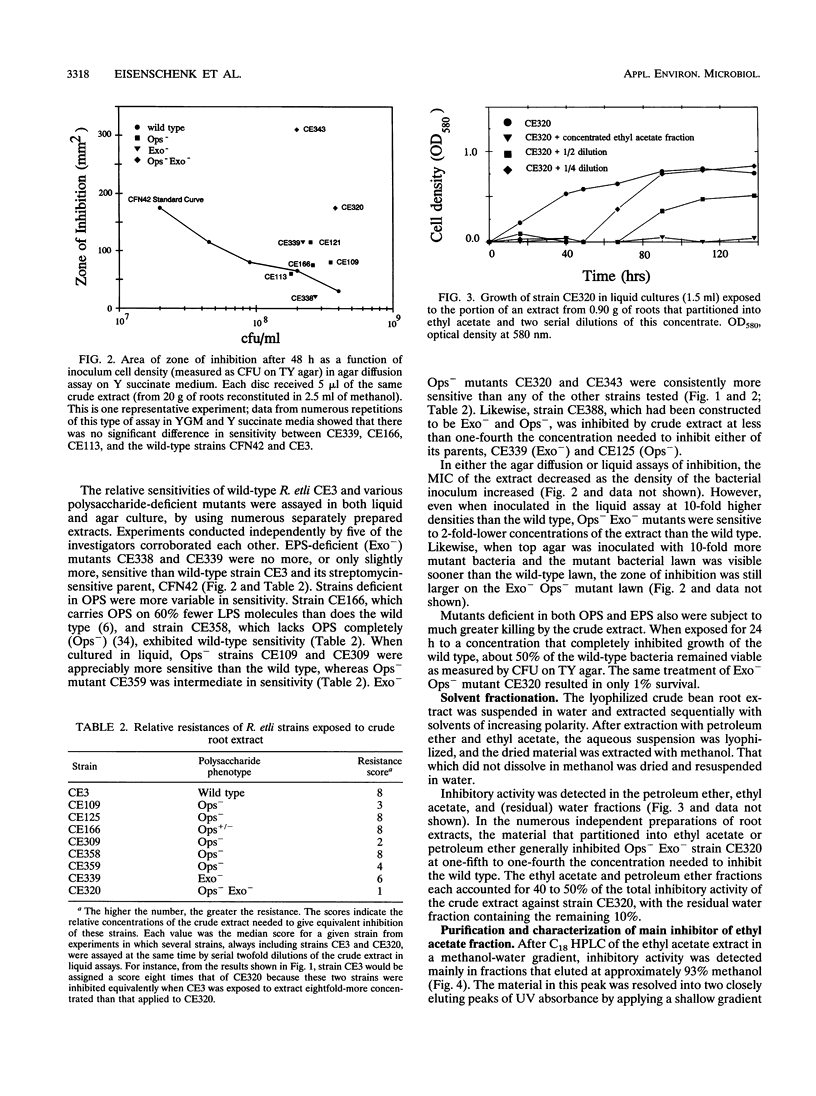
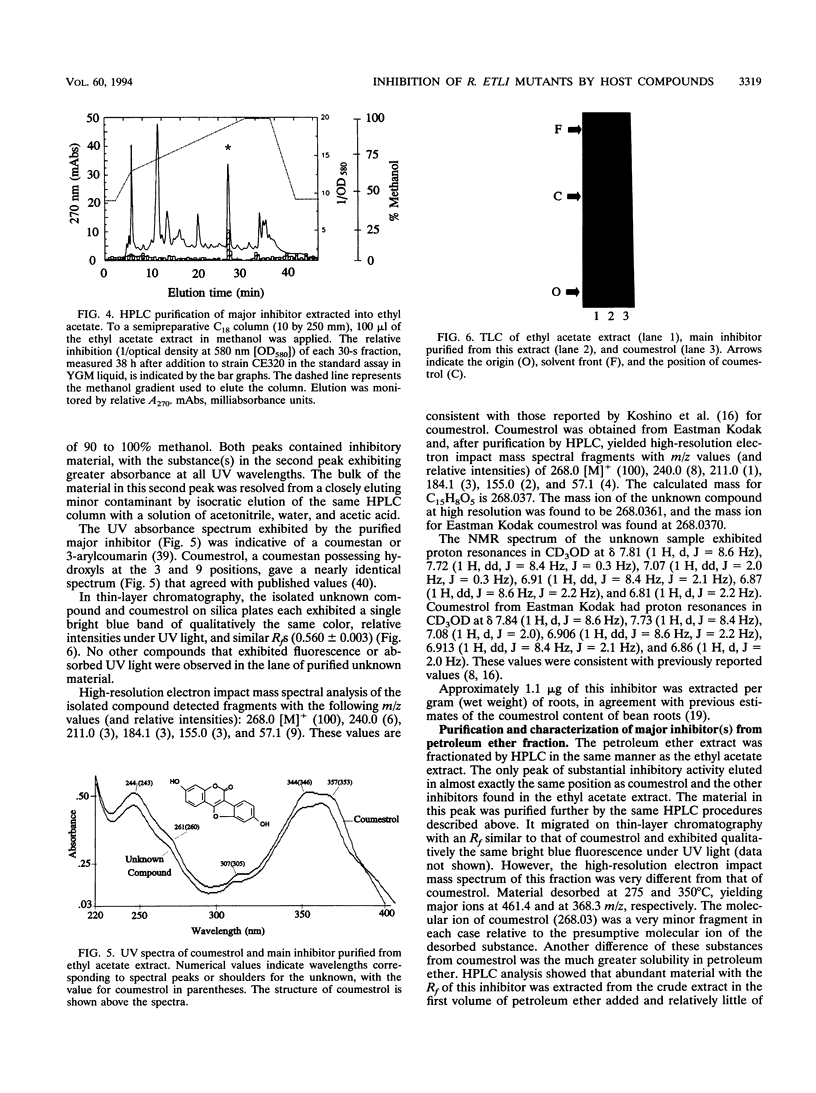



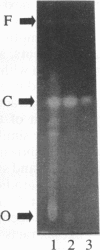
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brink B. A., Miller J., Carlson R. W., Noel K. D. Expression of Rhizobium leguminosarum CFN42 genes for lipopolysaccharide in strains derived from different R. leguminosarum soil isolates. J Bacteriol. 1990 Feb;172(2):548–555. doi: 10.1128/jb.172.2.548-555.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Garci F., Noel D., Hollingsworth R. The structures of the lipopolysaccharide core components from Rhizobium leguminosarum biovar phaseoli CE3 and two of its symbiotic mutants, CE109 and CE309. Carbohydr Res. 1989 Dec 21;195(1):101–110. doi: 10.1016/0008-6215(89)85092-x. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cava J. R., Elias P. M., Turowski D. A., Noel K. D. Rhizobium leguminosarum CFN42 genetic regions encoding lipopolysaccharide structures essential for complete nodule development on bean plants. J Bacteriol. 1989 Jan;171(1):8–15. doi: 10.1128/jb.171.1.8-15.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Truchet G. L., Hollingsworth R. I., Hrabak E. M., Pankratz H. S., Philip-Hollingsworth S., Salzwedel J. L., Chapman K., Appenzeller L., Squartini A. Rhizobium lipopolysaccharide modulates infection thread development in white clover root hairs. J Bacteriol. 1991 Sep;173(17):5371–5384. doi: 10.1128/jb.173.17.5371-5384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold R., Noel K. D. Rhizobium leguminosarum exopolysaccharide mutants: biochemical and genetic analyses and symbiotic behavior on three hosts. J Bacteriol. 1989 Sep;171(9):4821–4830. doi: 10.1128/jb.171.9.4821-4830.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr K. K., Guest D. I. Rapid, sensitive high-performance liquid chromatographic assay for isoflavonoids from cowpea (Vigna unguiculata). J Chromatogr. 1987 Jan 30;387:536–540. doi: 10.1016/s0021-9673(01)94567-4. [DOI] [PubMed] [Google Scholar]
- Gustafsson P., Nordström K., Normark S. Outer penetration barrier of Escherichia coli K-12: kinetics of the uptake of gentian violet by wild type and envelope mutants. J Bacteriol. 1973 Nov;116(2):893–900. doi: 10.1128/jb.116.2.893-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungria M., Joseph C. M., Phillips D. A. Rhizobium nod Gene Inducers Exuded Naturally from Roots of Common Bean (Phaseolus vulgaris L.). Plant Physiol. 1991 Oct;97(2):759–764. doi: 10.1104/pp.97.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuć J., Rush J. S. Phytoalexins. Arch Biochem Biophys. 1985 Feb 1;236(2):455–472. doi: 10.1016/0003-9861(85)90648-4. [DOI] [PubMed] [Google Scholar]
- Latchford J. W., Borthakur D., Johnston A. W. The products of Rhizobium genes, psi and pss, which affect exopolysaccharide production, are associated with the bacterial cell surface. Mol Microbiol. 1991 Sep;5(9):2107–2114. doi: 10.1111/j.1365-2958.1991.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Sanchez A., Fernandez L., Leemans J., Cevallos M. A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984 Apr;158(1):148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Vandenbosch K. A., Kulpaca B. Mutations in Rhizobium phaseoli that lead to arrested development of infection threads. J Bacteriol. 1986 Dec;168(3):1392–1401. doi: 10.1128/jb.168.3.1392-1401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankhurst C. E., Biggs D. R. Sensitivity of Rhizobium to selected isoflavonoids. Can J Microbiol. 1980 Apr;26(4):542–545. doi: 10.1139/m80-092. [DOI] [PubMed] [Google Scholar]
- Priefer U. B. Genes involved in lipopolysaccharide production and symbiosis are clustered on the chromosome of Rhizobium leguminosarum biovar viciae VF39. J Bacteriol. 1989 Nov;171(11):6161–6168. doi: 10.1128/jb.171.11.6161-6168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Gerhold D., Stacey G. Cell surface polysaccharides from Bradyrhizobium japonicum and a nonnodulating mutant. J Bacteriol. 1987 Jan;169(1):137–141. doi: 10.1128/jb.169.1.137-141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Matsuhashi M. Increase in sensitivity to antibiotics and lysozyme on deletion of lipopolysaccharides in Escherichia coli strains. J Bacteriol. 1973 Apr;114(1):453–454. doi: 10.1128/jb.114.1.453-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H., Brewin N. J., Noel K. D. Rhizobium leguminosarum CFN42 lipopolysaccharide antigenic changes induced by environmental conditions. J Bacteriol. 1992 Apr;174(7):2222–2229. doi: 10.1128/jb.174.7.2222-2229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully R. E., Terry M. E. Decreased Exopolysaccharide Synthesis by Anaerobic and Symbiotic Cells of Bradyrhizobium japonicum. Plant Physiol. 1985 Oct;79(2):445–450. doi: 10.1104/pp.79.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbosch K. A., Noel K. D., Kaneko Y., Newcomb E. H. Nodule initiation elicited by noninfective mutants of Rhizobium phaseoli. J Bacteriol. 1985 Jun;162(3):950–959. doi: 10.1128/jb.162.3.950-959.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R. A., Rao A. S., Mulders I. H., Goosen-de Roo L., van Loosdrecht M. C., Wijffelman C. A., Lugtenberg B. J. Isolation and characterization of mutants of Rhizobium leguminosarum bv. viciae 248 with altered lipopolysaccharides: possible role of surface charge or hydrophobicity in bacterial release from the infection thread. J Bacteriol. 1989 Feb;171(2):1143–1150. doi: 10.1128/jb.171.2.1143-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]