Abstract
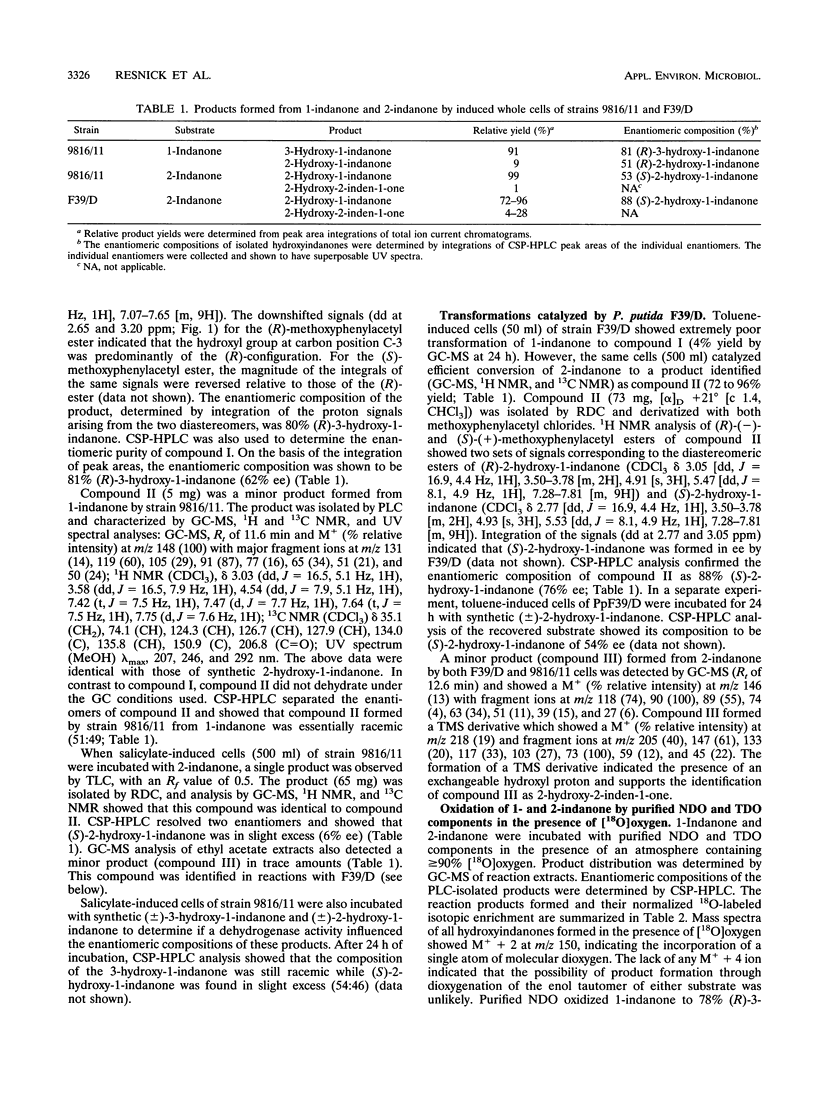
The biotransformation of 1-indanone and 2-indanone to hydroxyindanones was examined with bacterial strains expressing naphthalene dioxygenase (NDO) and toluene dioxygenase (TDO) as well as with purified enzyme components. Pseudomonas sp. strain 9816/11 cells, expressing NDO, oxidized 1-indanone to a mixture of 3-hydroxy-1-indanone (91%) and 2-hydroxy-1-indanone (9%). The (R)-3-hydroxy-1-indanone was formed in 62% enantiomeric excess (ee) (R:S, 81:19), while the 2-hydroxy-1-indanone was racemic. The same cells also formed 2-hydroxy-1-indanone from 2-indanone. Purified NDO components oxidized 1-indanone and 2-indanone to the same products produced by strain 9816/11. P. putida F39/D cells, expressing TDO, oxidized 2-indanone to (S)-2-hydroxy-1-indanone of 76% ee (R:S, 12:88) but did not oxidize 1-indanone efficiently. Purified TDO components also oxidized 2-indanone to (S)-2-hydroxy-1-indanone of 90% ee (R:S, 5:95) and failed to oxidize 1-indanone. Oxidation of 1- and 2-indanone in the presence of [18O]oxygen indicated that the hydroxyindanones were formed by the incorporation of a single atom of molecular oxygen (monooxygenation) rather than by the dioxygenation of enol tautomers of the ketone substrates. As alternatives to chemical synthesis, these biotransformations represent direct routes to 3-hydroxy-1-indanone and 2-hydroxy-1-indanone as the major products from 1-indanone and 2-indanone, respectively.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brand J. M., Cruden D. L., Zylstra G. J., Gibson D. T. Stereospecific hydroxylation of indan by Escherichia coli containing the cloned toluene dioxygenase genes from Pseudomonas putida F1. Appl Environ Microbiol. 1992 Oct;58(10):3407–3409. doi: 10.1128/aem.58.10.3407-3409.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Ratzkin B. J., Osslund T. D., Simon M. J., Wackett L. P., Gibson D. T. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science. 1983 Oct 14;222(4620):167–169. doi: 10.1126/science.6353574. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Hensley M., Yoshioka H., Mabry T. J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- Grifoll M., Casellas M., Bayona J. M., Solanas A. M. Isolation and characterization of a fluorene-degrading bacterium: identification of ring oxidation and ring fission products. Appl Environ Microbiol. 1992 Sep;58(9):2910–2917. doi: 10.1128/aem.58.9.2910-2917.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Jeffrey A. M., Gibson D. T. Cis-1,2-dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971 Jan;142(1):394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- Kobal V. M., Gibson D. T., Davis R. E., Garza A. X-ray determination of the absolute stereochemistry of the initial oxidation product formed from toluene by Pseudomonas puida 39-D. J Am Chem Soc. 1973 Jun 27;95(13):4420–4421. doi: 10.1021/ja00794a048. [DOI] [PubMed] [Google Scholar]
- Servé M. P., Ferry M. J., Yu K. O., Olson C. T., Hobson D. W. Metabolism and nephrotoxicity of indan in male Fischer 344 rats. J Toxicol Environ Health. 1990;29(4):409–416. doi: 10.1080/15287399009531401. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Suen W. C., Gibson D. T. Recombinant Escherichia coli strains synthesize active forms of naphthalene dioxygenase and its individual alpha and beta subunits. Gene. 1994 May 27;143(1):67–71. doi: 10.1016/0378-1119(94)90606-8. [DOI] [PubMed] [Google Scholar]
- Wackett L. P., Kwart L. D., Gibson D. T. Benzylic monooxygenation catalyzed by toluene dioxygenase from Pseudomonas putida. Biochemistry. 1988 Feb 23;27(4):1360–1367. doi: 10.1021/bi00404a041. [DOI] [PubMed] [Google Scholar]
- Yu K. O., Olson C. T., Ferry M. J., Serve M. P. Gas chromatographic/mass spectrometric studies of the urinary metabolites of male rats given indan. Biomed Environ Mass Spectrom. 1987 Nov;14(11):649–651. doi: 10.1002/bms.1200141114. [DOI] [PubMed] [Google Scholar]
- Zylstra G. J., Gibson D. T. Aromatic hydrocarbon degradation: a molecular approach. Genet Eng (N Y) 1991;13:183–203. doi: 10.1007/978-1-4615-3760-1_8. [DOI] [PubMed] [Google Scholar]



