Abstract
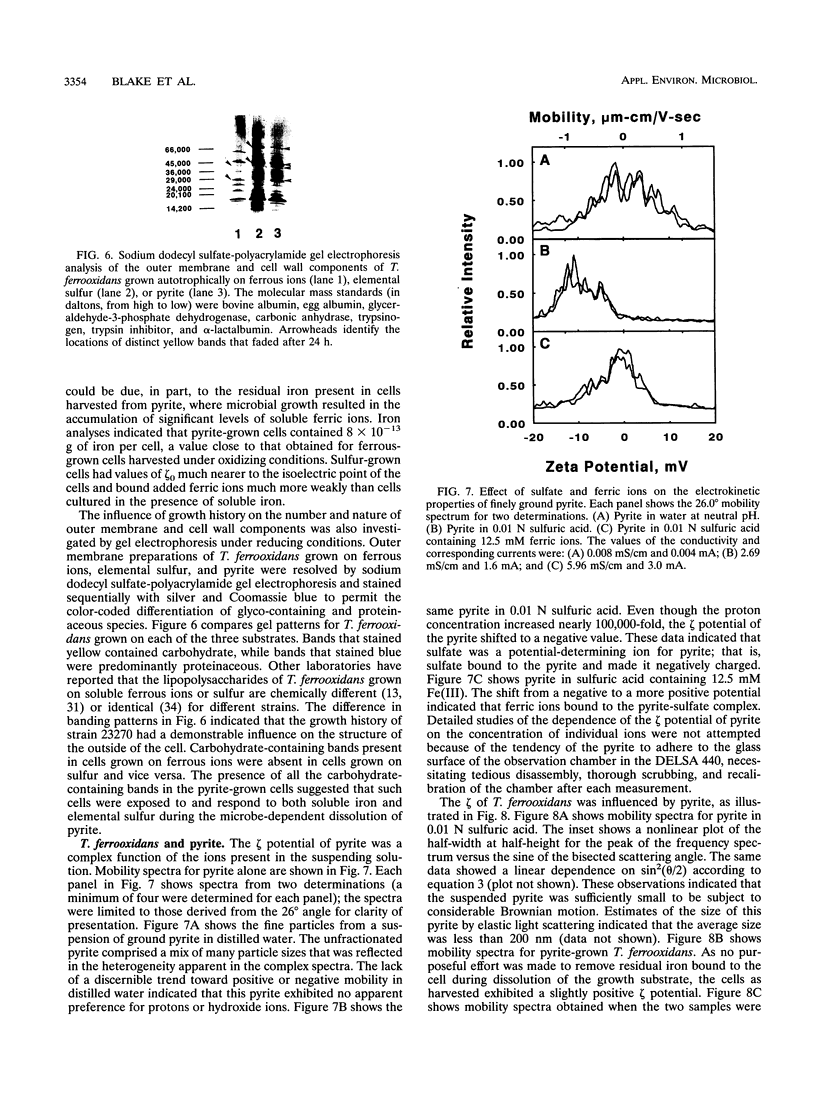
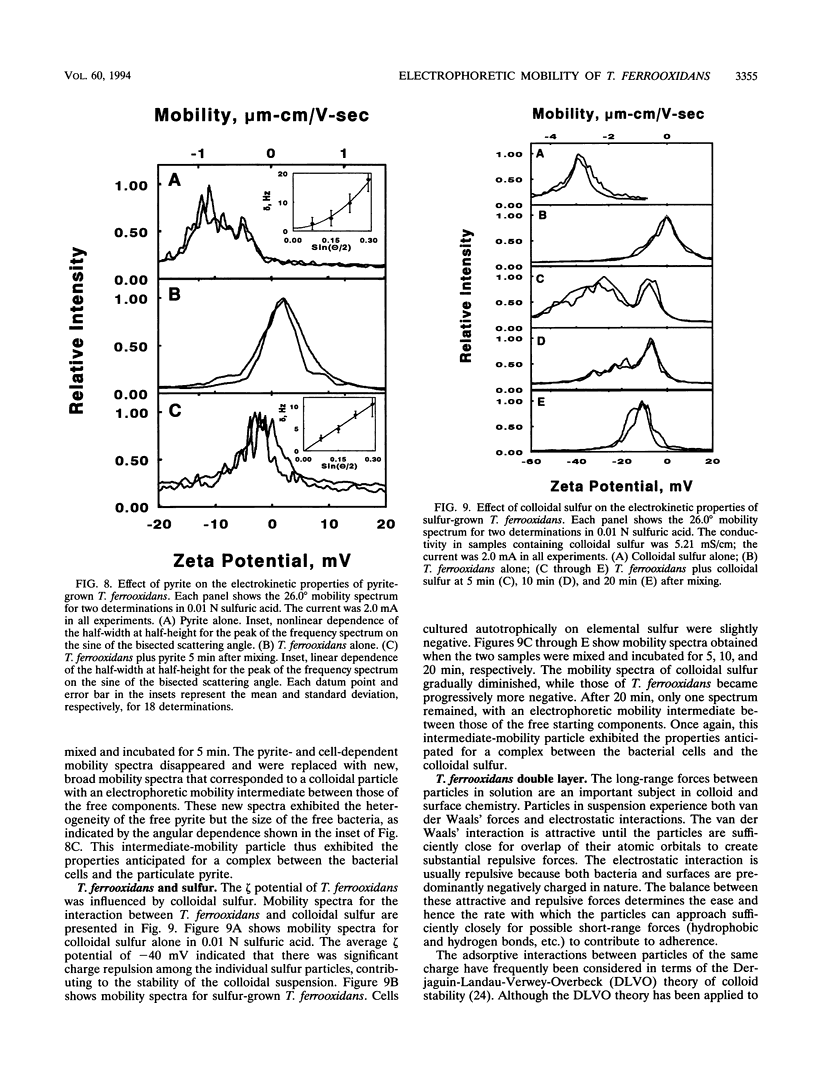
Thiobacillus ferroxidans is an obligate acidophile that respires aerobically on pyrite, elemental sulfur, or soluble ferrous ions. The electrophoretic mobility of the bacterium was determined by laser Doppler velocimetry under physiological conditions. When grown on pyrite or ferrous ions, washed cells were negatively charged at pH 2.0. The density of the negative charge depended on whether the conjugate base was sulfate, perchlorate, chloride, or nitrate. The addition of ferric ions shifted the net charge on the surface asymptotically to a positive value. When grown on elemental sulfur, washed cells were close to their isoelectric point at pH 2.0. Both pyrite and colloidal sulfur were negatively charged under the same conditions. The electrical double layer around the bacterial cells under physiological conditions exerted minimal electrostatic repulsion in possible interactions between the cell and either of its charged insoluble substrates. When Thiobacillus ferrooxidans was mixed with either pyrite or colloidal sulfur at pH 2.0, the mobility spectra of the free components disappeared with time to be replaced with a new colloidal particle whose electrophoretic properties were intermediate between those of the starting components. This new particle had the charge and size properties anticipated for a complex between the bacterium and its insoluble substrates. The utility of such measurements for the study of the interactions of chemolithotrophic bacteria with their insoluble substrates is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., VALENTINE R. C. Virus particle adsorption, III. Adsorption of viruses by cell monolayers and effects of some variables on adsorption. Biochim Biophys Acta. 1960 Jun 3;40:400–410. doi: 10.1016/0006-3002(60)91380-9. [DOI] [PubMed] [Google Scholar]
- Bennett J. C., Tributsch H. Bacterial leaching patterns on pyrite crystal surfaces. J Bacteriol. 1978 Apr;134(1):310–317. doi: 10.1128/jb.134.1.310-317.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R. C., Howard G. T., McGinness S. Enhanced yields of iron-oxidizing bacteria by in situ electrochemical reduction of soluble iron in the growth medium. Appl Environ Microbiol. 1994 Aug;60(8):2704–2710. doi: 10.1128/aem.60.8.2704-2710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins Y. E., Stotzky G. Heavy metals alter the electrokinetic properties of bacteria, yeasts, and clay minerals. Appl Environ Microbiol. 1992 May;58(5):1592–1600. doi: 10.1128/aem.58.5.1592-1600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devasia P., Natarajan K. A., Sathyanarayana D. N., Rao G. R. Surface Chemistry of Thiobacillus ferrooxidans Relevant to Adhesion on Mineral Surfaces. Appl Environ Microbiol. 1993 Dec;59(12):4051–4055. doi: 10.1128/aem.59.12.4051-4055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzandu J. K., Deh M. E., Barratt D. L., Wise G. E. Detection of erythrocyte membrane proteins, sialoglycoproteins, and lipids in the same polyacrylamide gel using a double-staining technique. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1733–1737. doi: 10.1073/pnas.81.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Romero P. Growth of Thiobacillus ferrooxidans on Elemental Sulfur. Appl Environ Microbiol. 1987 Aug;53(8):1907–1912. doi: 10.1128/aem.53.8.1907-1912.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt W. E., Vestal J. R. Physical and chemical studies of Thiobacillus ferroxidans lipopolysaccharides. J Bacteriol. 1975 Aug;123(2):642–650. doi: 10.1128/jb.123.2.642-650.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura N., Kitamura K., Saiki H. Selective Adhesion of Thiobacillus ferrooxidans to Pyrite. Appl Environ Microbiol. 1993 Dec;59(12):4044–4050. doi: 10.1128/aem.59.12.4044-4050.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro T., Stotzky G. Sorption between microorganisms and clay minerals as determined by the electrical sensing zone particle analyser. Can J Microbiol. 1968 Apr;14(4):299–307. doi: 10.1139/m68-049. [DOI] [PubMed] [Google Scholar]
- Tuovinen O. H., Kelly D. P. Studies on the growth of Thiobacillus ferrooxidans. I. Use of membrane filters and ferrous iron agar to determine viable numbers, and comparison with 14 CO 2 -fixation and iron oxidation as measures of growth. Arch Mikrobiol. 1973;88(4):285–298. [PubMed] [Google Scholar]
- Vestal J. R., Lundgren D. G., Milner K. C. Toxic and immunological differences among lipopolysaccharides from Thiobacillus ferrooxidans grown autotrophically and heterotrophically. Can J Microbiol. 1973 Nov;19(11):1335–1339. doi: 10.1139/m73-215. [DOI] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Zehnder A. J. Influence of interfaces on microbial activity. Microbiol Rev. 1990 Mar;54(1):75–87. doi: 10.1128/mr.54.1.75-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]