Abstract
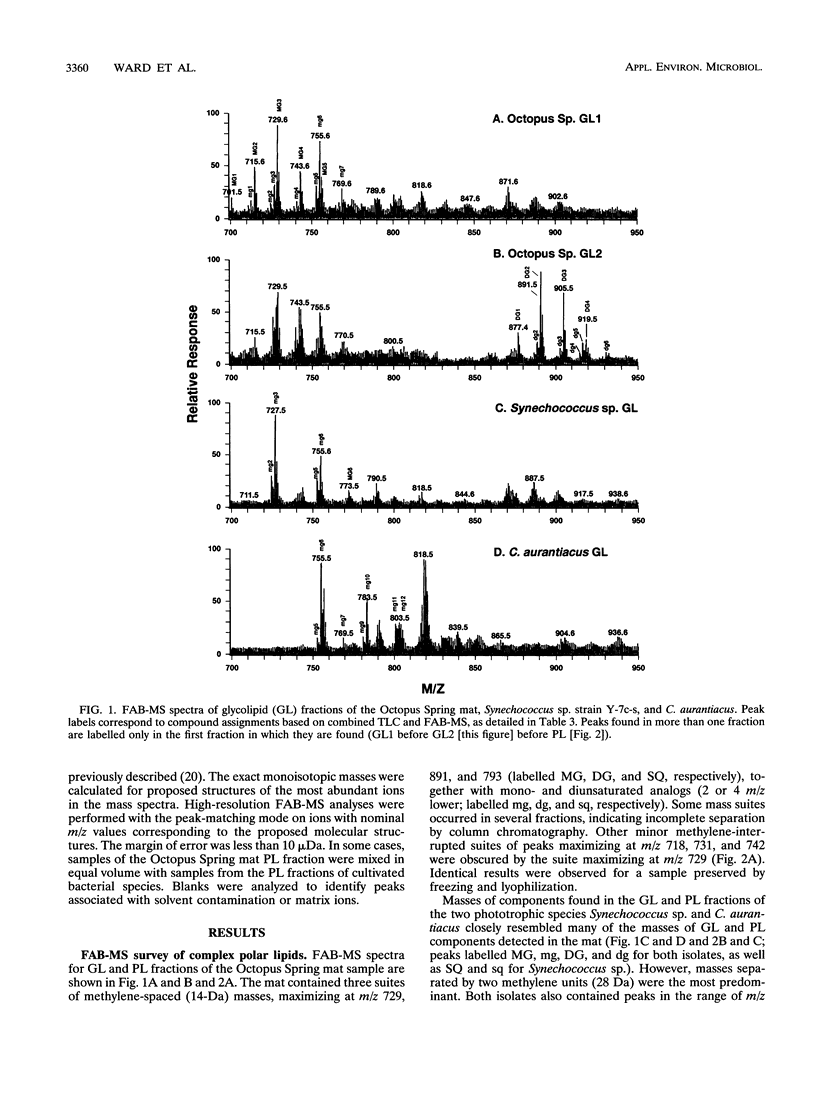
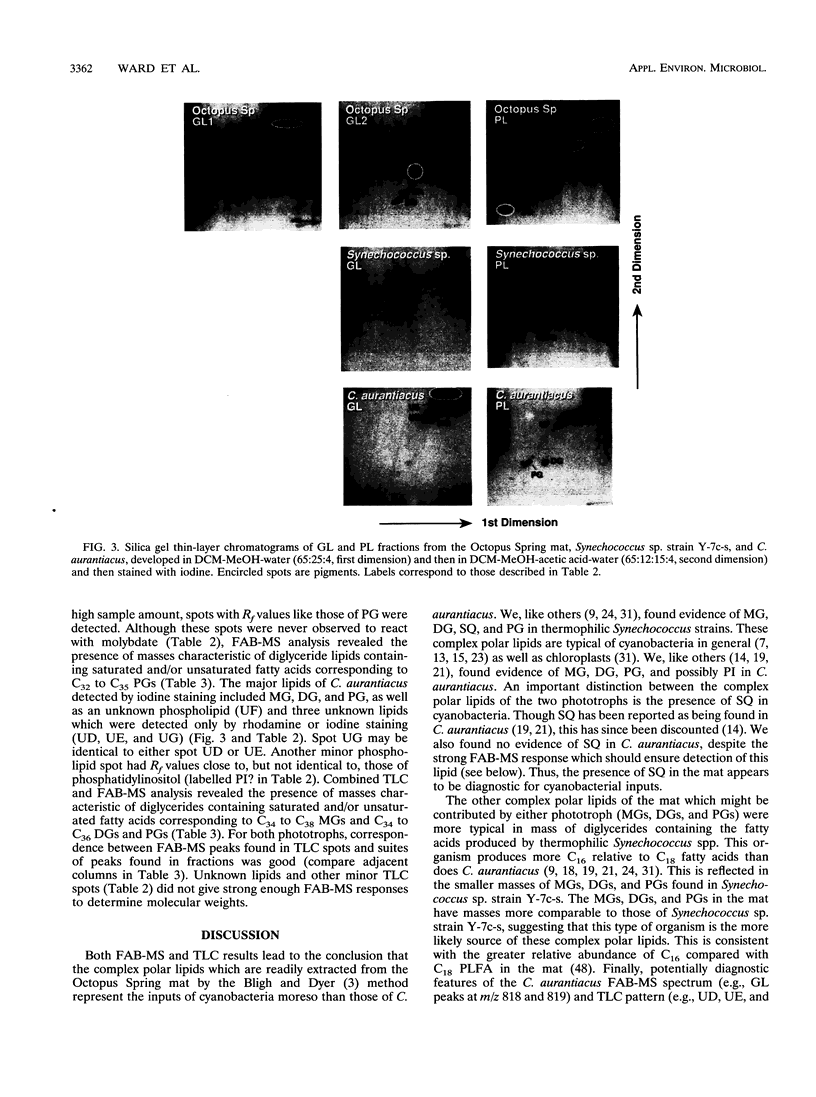
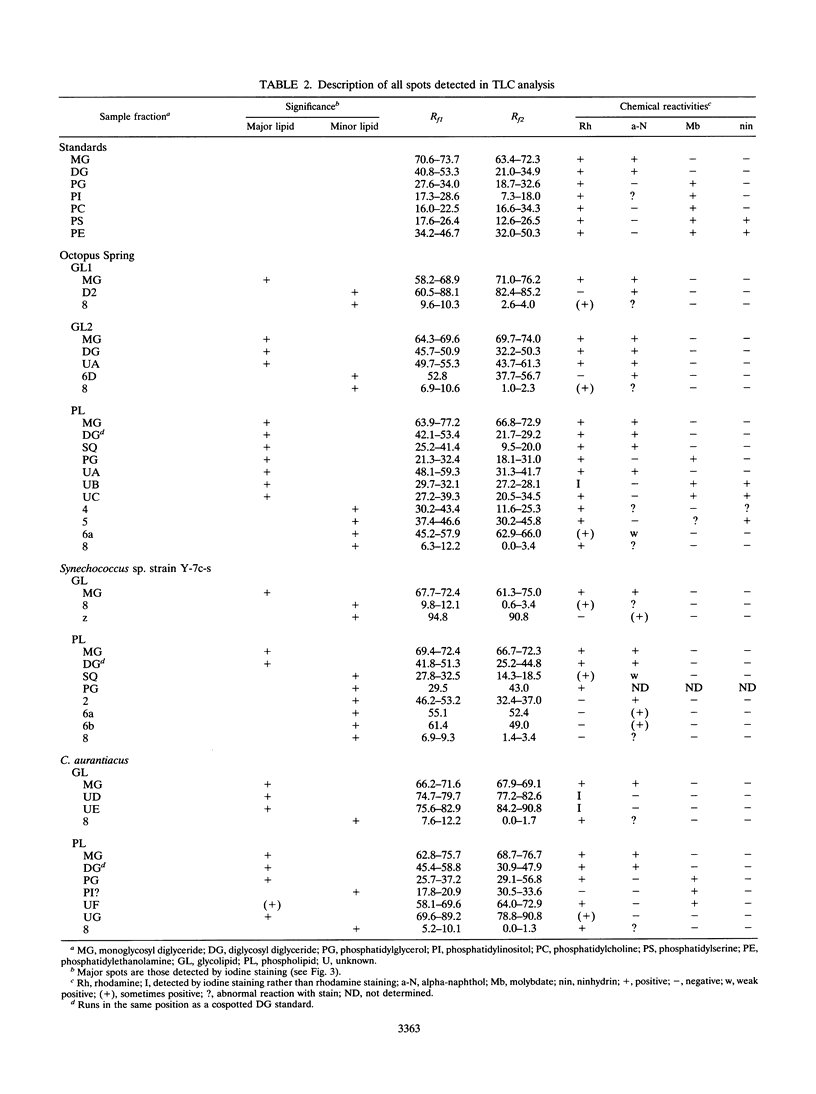
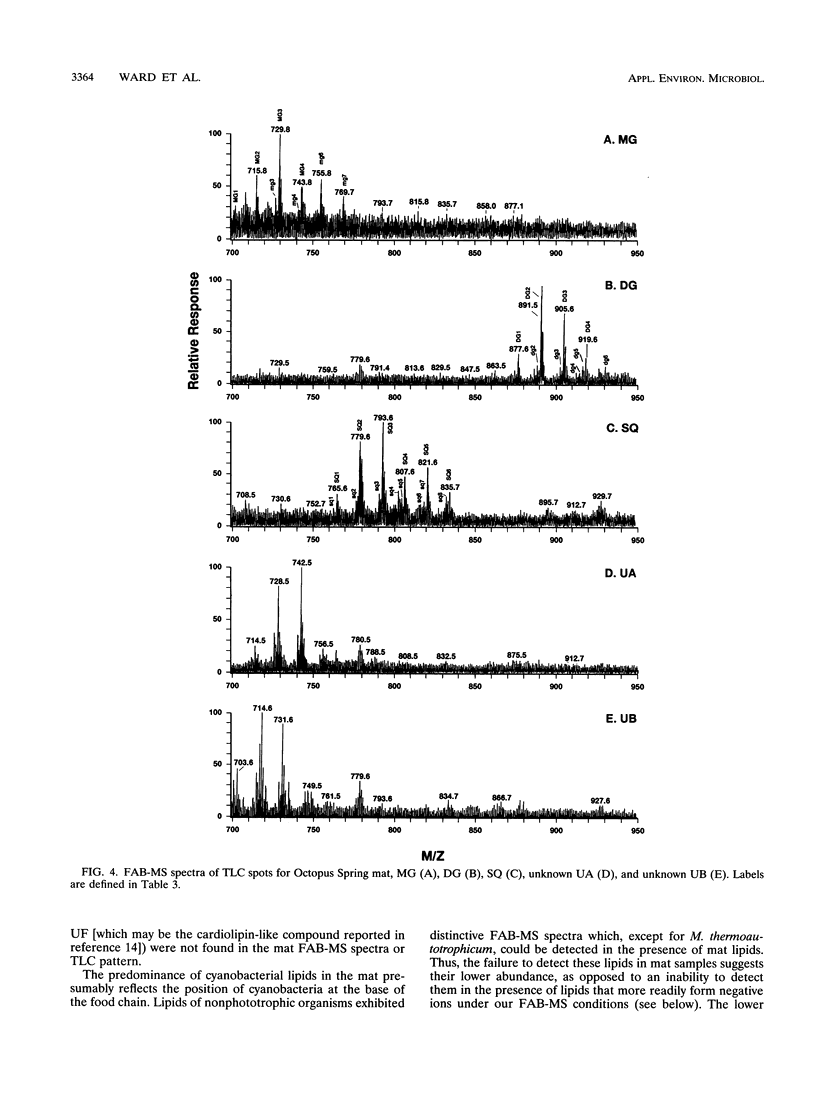
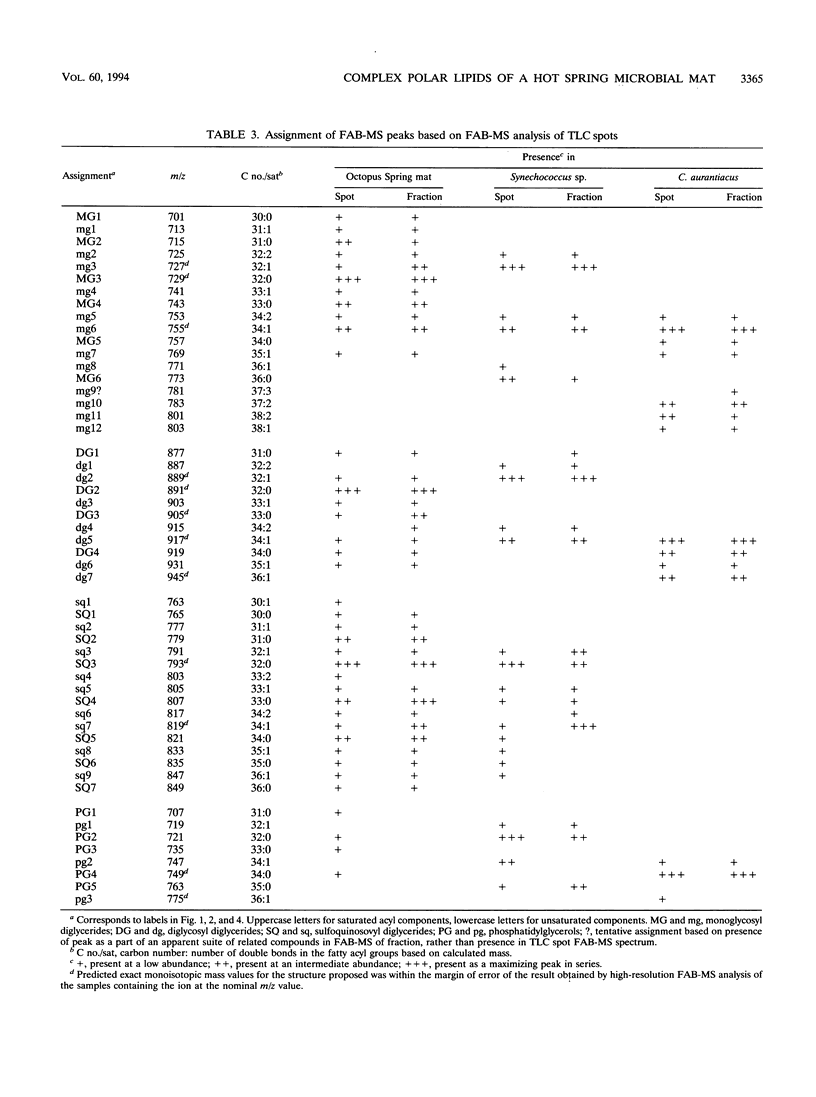
The complex polar lipids of the hot spring cyanobacterial mat in the 50 to 55 degrees C region of Octopus Spring, Yellowstone National Park, and of thermophilic bacteria cultivated from this or similar habitats, were compared in an attempt to understand the microbial sources of the major lipid biomarkers in this community. Intact complex lipids were analyzed directly by fast atom bombardment mass spectrometry (FAB-MS), two-dimensional thin-layer chromatography (TLC), and combined TLC-FAB-MS. FAB-MS and TLC gave qualitatively similar results, suggesting that the mat contains major lipids most like those of the cyanobacterial isolate we studied, Synechococcus sp. strain Y-7c-s. These include monoglycosyl, diglycosyl, and sulfoquinosovyl diglycerides (MG, DG, and SQ, respectively) and phosphatidyl glycerol (PG). Though Chloroflexus aurantiacus also contains MG, DG, and PG, the fatty acid chain lengths of mat MGs, DGs, and PGs resemble more those of cyanobacterial than green nonsulfur bacterial lipids. FAB-MS spectra of the lipids of nonphototrophic bacterial isolates were distinctively different from those of the mat and phototrophic isolates. The lipids of these nonphototrophic isolates were not detected in the mat, but most could be detected when added to mat samples. The mat also contains major glycolipids and aminophospholipids of unknown structure and origin. FAB-MS and TLC did not always give quantitatively similar results. In particular, PG and SQ may give disproportionately high FAB-MS responses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Freeze H. Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol. 1969 Apr;98(1):289–297. doi: 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fork D. C. Effect of Growth Temperature on the Lipid and Fatty Acid Composition, and the Dependence on Temperature of Light-induced Redox Reactions of Cytochrome f and of Light Energy Redistribution in the Thermophilic Blue-Green Alga Synechococcus lividus. Plant Physiol. 1979 Mar;63(3):524–530. doi: 10.1104/pp.63.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huflejt M. E., Tremolieres A., Pineau B., Lang J. K., Hatheway J., Packer L. Changes in membrane lipid composition during saline growth of the fresh water cyanobacterium Synechococcus 6311. Plant Physiol. 1990;94:1512–1521. doi: 10.1104/pp.94.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens U. J., Weckesser J. The fine structure and chemical composition of the cell wall and sheath layers of cyanobacteria. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):41–44. doi: 10.1016/s0769-2609(85)80019-3. [DOI] [PubMed] [Google Scholar]
- Kallas T., Castenholz R. W. Internal pH and ATP-ADP pools in the cyanobacterium Synechococcus sp. during exposure to growth-inhibiting low pH. J Bacteriol. 1982 Jan;149(1):229–236. doi: 10.1128/jb.149.1.229-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. N. Fatty acid composition of unicellular strains of blue-green algae. J Bacteriol. 1972 Feb;109(2):827–834. doi: 10.1128/jb.109.2.827-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. N., Gray A. M. Preliminary analysis of lipids and fatty acids of green bacteria and Chloroflexus aurantiacus. J Bacteriol. 1974 Oct;120(1):131–138. doi: 10.1128/jb.120.1.131-138.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel K. D., Fredrickson H. L. Fast atom bombardment mass spectrometry as a rapid means of screening mixtures of ether-linked polar lipids from extremely halophilic archaebacteria for the presence of novel chemical structures. J Chromatogr. 1991 Jan 2;562(1-2):369–376. doi: 10.1016/0378-4347(91)80592-z. [DOI] [PubMed] [Google Scholar]
- Miller M., Pedersen J. Z., Cox R. P. Effect of growth temperature on membrane dynamics in a thermophilic cyanobacterium: a spin label study. Biochim Biophys Acta. 1988 Sep 1;943(3):501–510. doi: 10.1016/0005-2736(88)90383-5. [DOI] [PubMed] [Google Scholar]
- Pierson B. K., Castenholz R. W. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. and sp. nov. Arch Microbiol. 1974;100(1):5–24. doi: 10.1007/BF00446302. [DOI] [PubMed] [Google Scholar]
- Ruff-Roberts A. L., Kuenen J. G., Ward D. M. Distribution of cultivated and uncultivated cyanobacteria and Chloroflexus-like bacteria in hot spring microbial mats. Appl Environ Microbiol. 1994 Feb;60(2):697–704. doi: 10.1128/aem.60.2.697-704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Walraven H. S., Koppenaal E., Marvin H. J., Hagendoorn M. J., Kraayenhof R. Lipid specificity for the reconstitution of well-coupled ATPase proteoliposomes and a new method for lipid isolation from photosynthetic membranes. Eur J Biochem. 1984 Nov 2;144(3):563–569. doi: 10.1111/j.1432-1033.1984.tb08502.x. [DOI] [PubMed] [Google Scholar]
- Ward D. M., Weller R., Bateson M. M. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature. 1990 May 3;345(6270):63–65. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]
- Weller R., Bateson M. M., Heimbuch B. K., Kopczynski E. D., Ward D. M. Uncultivated cyanobacteria, Chloroflexus-like inhabitants, and spirochete-like inhabitants of a hot spring microbial mat. Appl Environ Microbiol. 1992 Dec;58(12):3964–3969. doi: 10.1128/aem.58.12.3964-3969.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller R., Weller J. W., Ward D. M. 16S rRNA sequences of uncultivated hot spring cyanobacterial mat inhabitants retrieved as randomly primed cDNA. Appl Environ Microbiol. 1991 Apr;57(4):1146–1151. doi: 10.1128/aem.57.4.1146-1151.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]




