Abstract
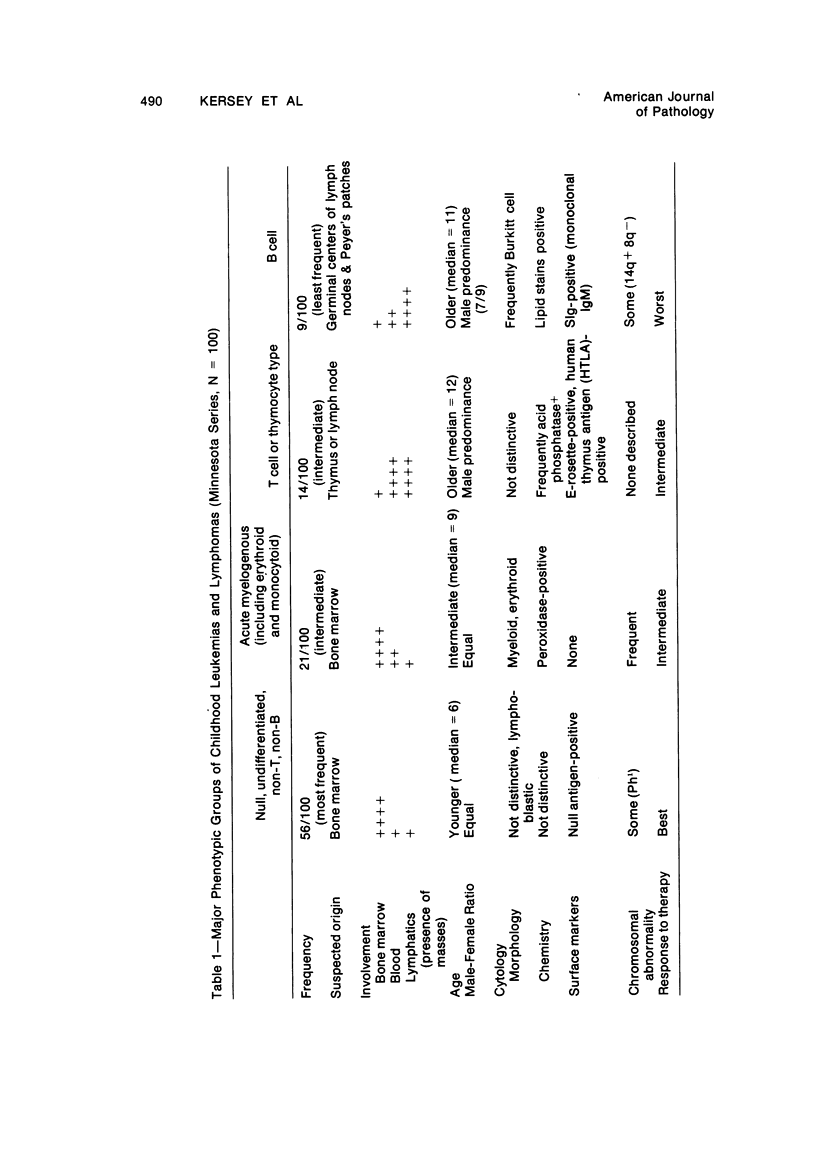
One hundred consecutive newly diagnosed cases of leukemia and lymphoma in children from 0 to 16 years of age presenting at the University of Minnesota from 1973 to 1977 were studied. Clinical features were correlated with phenotypic features of blast cells, including surface markers and cytomorphology. Four groups with distinct clinical and pathologic features emerged from the study: a) The acute leukemias of the “null” or “undifferentiated” group were those in which the malignant cells carried distinctive null leukemia surface antigen and lacked features of either T cells (E-rosette positivity) or B cells (surface immunoglobulin positivity). These cases occurred most frequently in the series (56% of total cases), peaked in incidence at 6 years, were associated with extensive bone marrow involvement, lacked distinguishing cytomorphologic features, and had the best response to therapy of all groups. b) The acute myelogenous leukemias, including those with myeloid, monocytoid, or erythroid features or a combination of the above, had extensive bone marrow involvement and the characteristic morphology. This group was seen with intermediate frequency and showed an intermediate response to therapy. c) Leukemia-lymphomas of the T-cell group were frequently associated with mediastinal masses and other masses, a cytomorphology which was different from the B-cell group but similar to the null group, and high white cell counts. These cases occurred with intermediate frequency (14%) and had a worse prognosis than the null group. d) Leukemia-lymphomas of the B-cell group had monoclonal surface immunoglobulin with μ-heavy and either κ or λ light chain. These patients were least frequent in the series, frequently presented with abdominal masses, and had a characteristic Burkitt cell morphology. Prognosis was the worst of all patients in our series. These data suggest that the major phenotypic groups of childhood leukemia and lymphoma have differing prognoses and should receive differing forms of therapy. Clinical and pathologic features of each group are sufficiently distinctive to suggest that they may have different causes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr R. D., Whang-Peng J., Perry S. Hemopoietic stem cells in human peripheral blood. Science. 1975 Oct 17;190(4211):284–285. doi: 10.1126/science.1179209. [DOI] [PubMed] [Google Scholar]
- Billing R., Rafizadeh B., Drew I., Hartman G., Gale R., Terasaki P. Human B-lymphocyte antigens expressed by lymphocytic and myelocytic leukemia cells. I. Detection by rabbit antisera. J Exp Med. 1976 Jul 1;144(1):167–178. doi: 10.1084/jem.144.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia P. F., Kersey J. H., Gajil-Peczalska K. J., Krivit W., Nesbit M. E. Prognostic significance of surface marker analysis in childhood non-Hodgkin's lymphoproliferative malifnancies. Am J Hematol. 1976;1(4):405–417. doi: 10.1002/ajh.2830010406. [DOI] [PubMed] [Google Scholar]
- Flandrin G., Brouet J. C., Daniel M. T., Preud'homme J. L. Acute leukemia with Burkitt's tumor cells: A study of six cases with special reference to lymphocyte surface markers. Blood. 1975 Feb;45(2):183–188. [PubMed] [Google Scholar]
- Gajl-Peczalska K. J., Chartrand S., Bloomfield C. D., Corte J., Coccia P. F., Nesbit M. E., Kersey J. H. Lymphocytes bearing receptors for both sheep erythrocytes and complement in patients with neoplastic and non-neoplastic diseases. Clin Immunol Immunopathol. 1977 Sep;8(2):292–299. doi: 10.1016/0090-1229(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Ravindranath Y., Peterson W. D., Jr T and B lymphocyte antigen-positive null cell leukemias. Blood. 1977 Mar;49(3):371–378. [PubMed] [Google Scholar]
- Kersey J. H., Gajl-Peczalska J. T and B lymphocytes in humans. A review. Am J Pathol. 1975 Nov;81(2):446–458. [PMC free article] [PubMed] [Google Scholar]
- Kersey J., Nesbit M., Hallgren H., Sabad A., Yunis E., Gajl-Peczalska K. Evidence for origin of certain childhood acute lymphoblastic leukemias and lymphomas in thymus-derived lymphocytes. Cancer. 1975 Oct;36(4):1348–1352. doi: 10.1002/1097-0142(197510)36:4<1348::aid-cncr2820360424>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Mann R. B., Jaffe E. S., Braylan R. C., Nanba K., Frank M. M., Ziegler J. L., Berard C. W. Non-endemic Burkitts's lymphoma. A B-cell tumor related to germinal centers. N Engl J Med. 1976 Sep 23;295(13):685–691. doi: 10.1056/NEJM197609232951301. [DOI] [PubMed] [Google Scholar]
- McKenna R. W., Parkin J., Kersey J. H., Gajl-Peczalska K. J., Peterson L., Brunning R. D. Chronic lymphoproliferative disorder with unusual clinical, morphologic, ultrastructural and membrane surface marker characteristics. Am J Med. 1977 Apr;62(4):588–596. doi: 10.1016/0002-9343(77)90422-3. [DOI] [PubMed] [Google Scholar]
- Sen L., Borella L. Clinical importance of lymphoblasts with T markers in childhood acute leukemia. N Engl J Med. 1975 Apr 17;292(16):828–832. doi: 10.1056/NEJM197504172921604. [DOI] [PubMed] [Google Scholar]
- Tsukimoto I., Wong K. Y., Lampkin B. C. Surface markers and prognostic factors in acute lymphoblastic leukemia. N Engl J Med. 1976 Jan 29;294(5):245–248. doi: 10.1056/NEJM197601292940503. [DOI] [PubMed] [Google Scholar]



