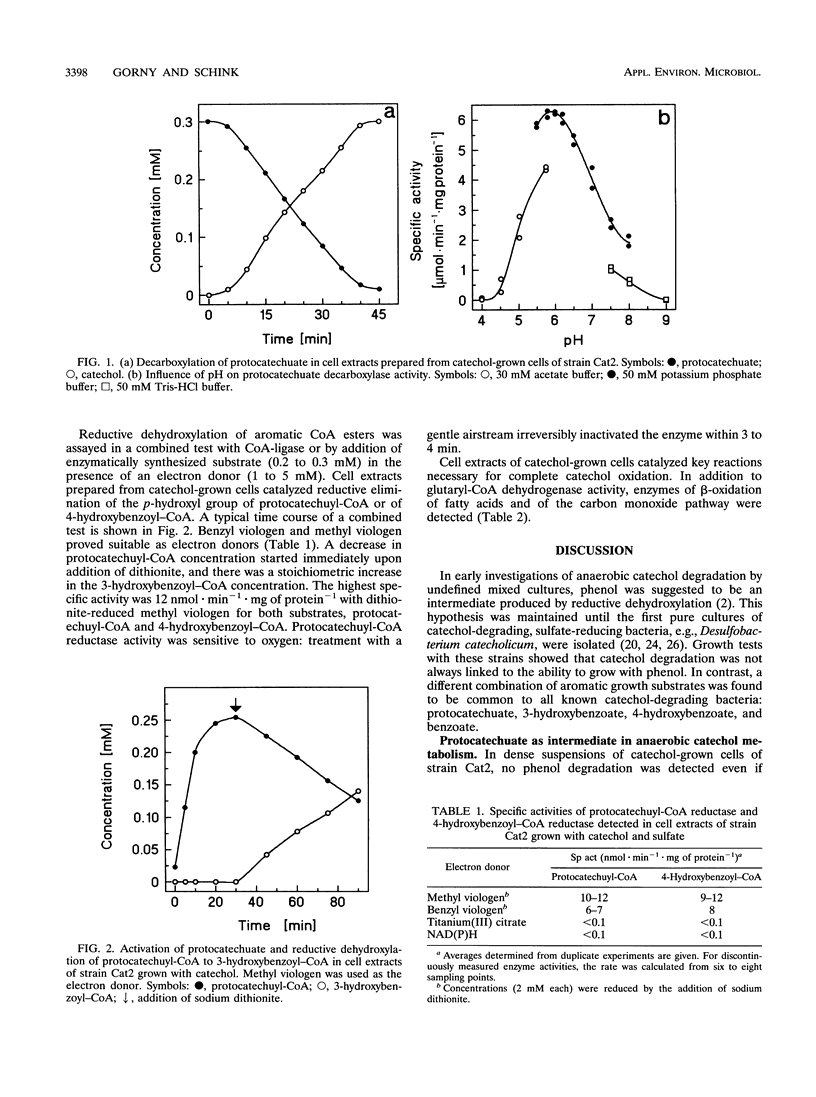
Abstract
Under anoxic conditions, most methoxylated mononuclear aromatic compounds are degraded by bacteria, with catechol being formed as an important intermediate. On the basis of our experiments with the sulfate-reducing bacterium Desulfobacterium sp. strain Cat2, we describe for the first time the enzymatic activities involved in the complete anaerobic oxidation of catechol and protocatechuate. Results obtained from experiments with dense cell suspensions of strain Cat2 demonstrated that all enzymes necessary for protocatechuate and benzoate degradation were induced during growth with catechol. In addition, anaerobic oxidation of catechol was found to be a CO2-dependent process. Phenol was not degraded in suspensions of cells grown with catechol. In cell extracts of Desulfobacterium sp. strain Cat2, protocatechuyl-coenzyme A (CoA) was formed from catechol, bicarbonate, and uncombined CoA. This oxygen-sensitive reaction requires high concentrations of both bicarbonate and protein, and only very low levels of enzyme were detected. In a second oxygen-sensitive step, protocatechuyl-CoA was reduced to 3-hydroxybenzoyl-CoA by reductive elimination of the p-hydroxyl group. Further dehydroxylation to benzoyl-CoA was not detectable. Key reactions described for anaerobic degradation of benzoate were catalyzed by cell extracts of strain Cat2, too.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balba M. T., Evans W. C. The methanogenic biodegradation of catechol by a microbial consortium: evidence for the production of phenol through cis-benzenediol. Biochem Soc Trans. 1980 Aug;8(4):452–453. doi: 10.1042/bst0080452. [DOI] [PubMed] [Google Scholar]
- Brackmann R., Fuchs G. Enzymes of anaerobic metabolism of phenolic compounds. 4-Hydroxybenzoyl-CoA reductase (dehydroxylating) from a denitrifying Pseudomonas species. Eur J Biochem. 1993 Apr 1;213(1):563–571. doi: 10.1111/j.1432-1033.1993.tb17795.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brune A., Schink B. Pyrogallol-to-phloroglucinol conversion and other hydroxyl-transfer reactions catalyzed by cell extracts of Pelobacter acidigallici. J Bacteriol. 1990 Feb;172(2):1070–1076. doi: 10.1128/jb.172.2.1070-1076.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert G. B., Thauer R. K. Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol. 1978 Nov;136(2):597–606. doi: 10.1128/jb.136.2.597-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy J. B., Young L. Y. Anaerobic biodegradation of eleven aromatic compounds to methane. Appl Environ Microbiol. 1979 Jul;38(1):84–89. doi: 10.1128/aem.38.1.84-89.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heising S., Brune A., Schink B. Anaerobic degradation of 3-hydroxybenzoate by a newly isolated nitrate-reducing bacterium. FEMS Microbiol Lett. 1991 Dec 1;68(3):267–272. doi: 10.1016/0378-1097(91)90367-j. [DOI] [PubMed] [Google Scholar]
- Härtel U., Eckel E., Koch J., Fuchs G., Linder D., Buckel W. Purification of glutaryl-CoA dehydrogenase from Pseudomonas sp., an enzyme involved in the anaerobic degradation of benzoate. Arch Microbiol. 1993;159(2):174–181. doi: 10.1007/BF00250279. [DOI] [PubMed] [Google Scholar]
- Kuever J., Kulmer J., Jannsen S., Fischer U., Blotevogel K. H. Isolation and characterization of a new spore-forming sulfate-reducing bacterium growing by complete oxidation of catechol. Arch Microbiol. 1993;159(3):282–288. doi: 10.1007/BF00248485. [DOI] [PubMed] [Google Scholar]
- Lack A., Fuchs G. Carboxylation of phenylphosphate by phenol carboxylase, an enzyme system of anaerobic phenol metabolism. J Bacteriol. 1992 Jun;174(11):3629–3636. doi: 10.1128/jb.174.11.3629-3636.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel S. M., Eberhard A. E., Gibson J., Harwood C. S. Involvement of coenzyme A thioesters in anaerobic metabolism of 4-hydroxybenzoate by Rhodopseudomonas palustris. J Bacteriol. 1989 Jan;171(1):1–7. doi: 10.1128/jb.171.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell S., Bak F., Pfennig N. Anaerobic degradation of aniline and dihydroxybenzenes by newly isolated sulfate-reducing bacteria and description of Desulfobacterium anilini. Arch Microbiol. 1989;152(6):556–563. doi: 10.1007/BF00425486. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



