Abstract
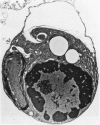
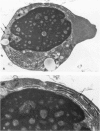
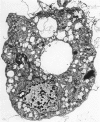
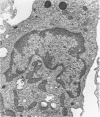
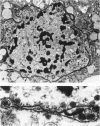
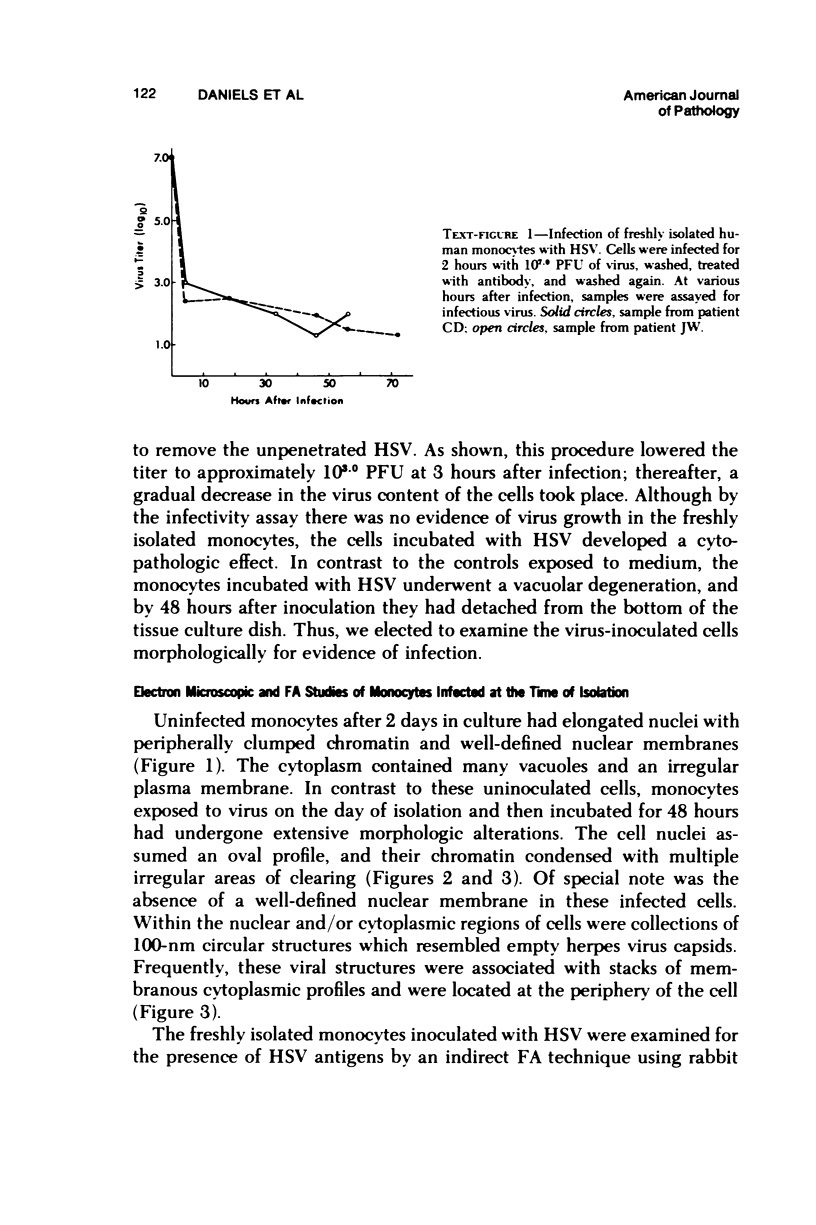
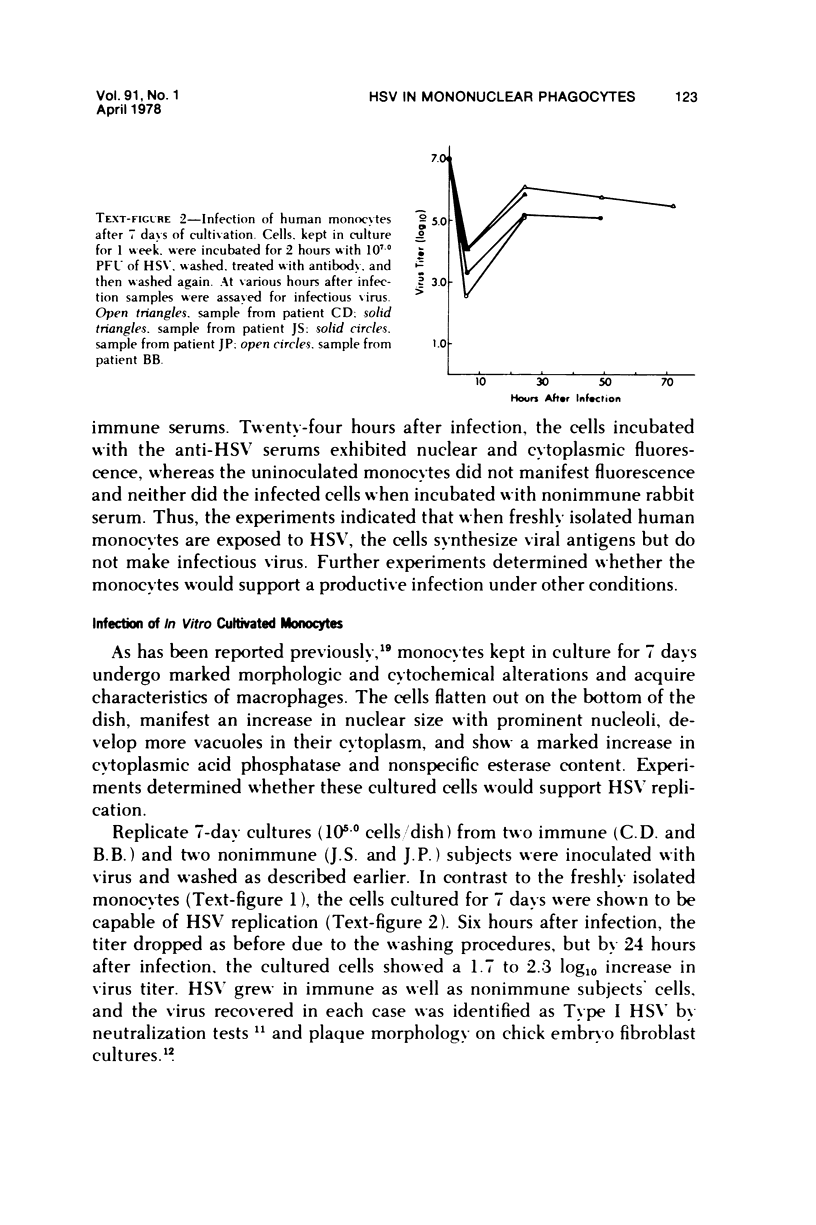
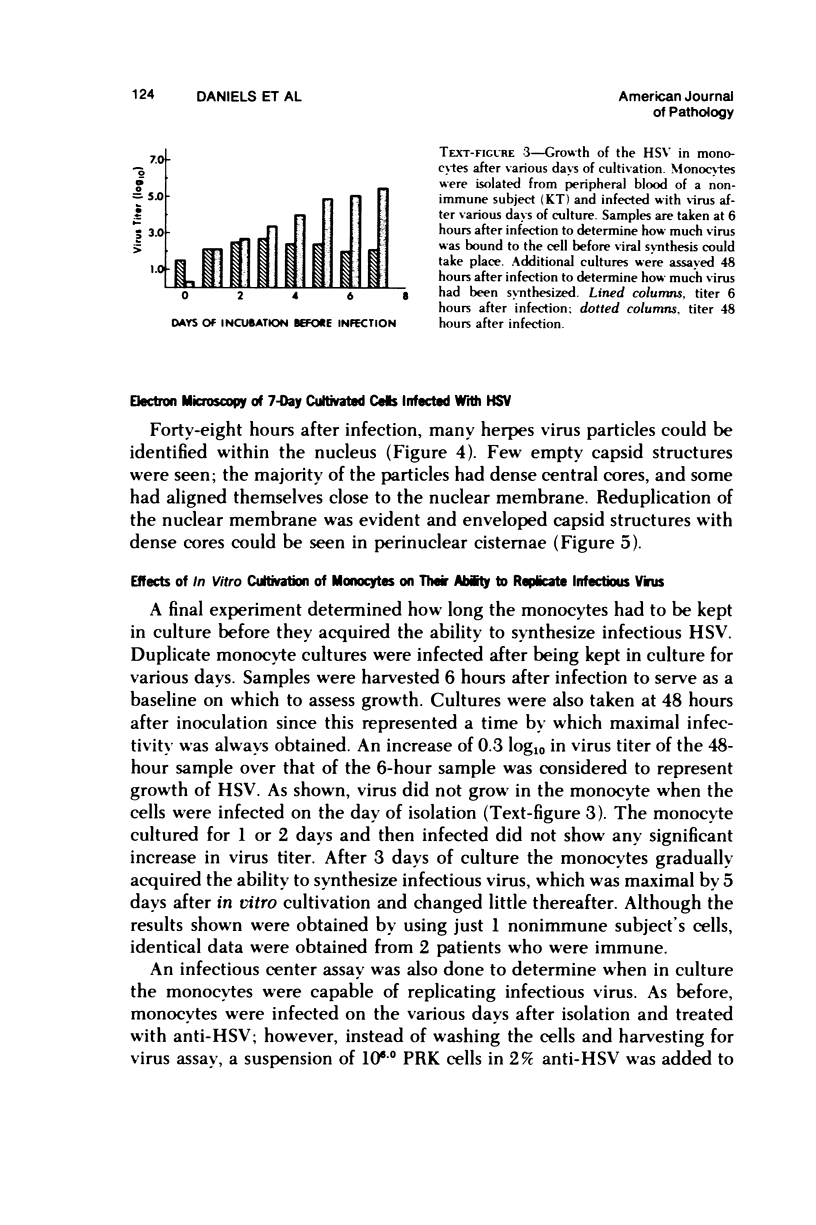
The ability of Type I herpes simplex (HSV) to replicate in normal human monouclear phagocytes was investigated. Mononuclear leukocytes were obtained from the peripheral blood of patients by Ficoll-Hypaque gradient centrifugation, and the monocytes were isolated by allowing the cells to adhere to tissue culture dishes. The monocytes (10(5.0) cells) were infected (10(7.0) PFU HSV) either immediately after isolation or were cultured in vitro for varying numbers of days and were then infected. Inoculation of freshly isolated monocytes resulted primarily in an abortive infection. HSV antigens were produced by the cells, as determined by a indirect fluorescent antibody technique, and empty herpes capsid structures were detected by electron microscopy of the inoculated monocytes; however, no increase in virus titer was noted in the cultures. Inoculation of viable cells that had been maintained for 7 days in culture resulted in a productive infection. An increase in titer was noted 24 hours after inoculation, and normal virus maturation was documented by ultrastructural study of the infected cells. The experiments show that the interaction of HSV with human mononuclear phagocytes is complex, and the data suggest that whether or not the cell replicates infectious virus may depend on the functional activity of the cell.
Full text
PDF








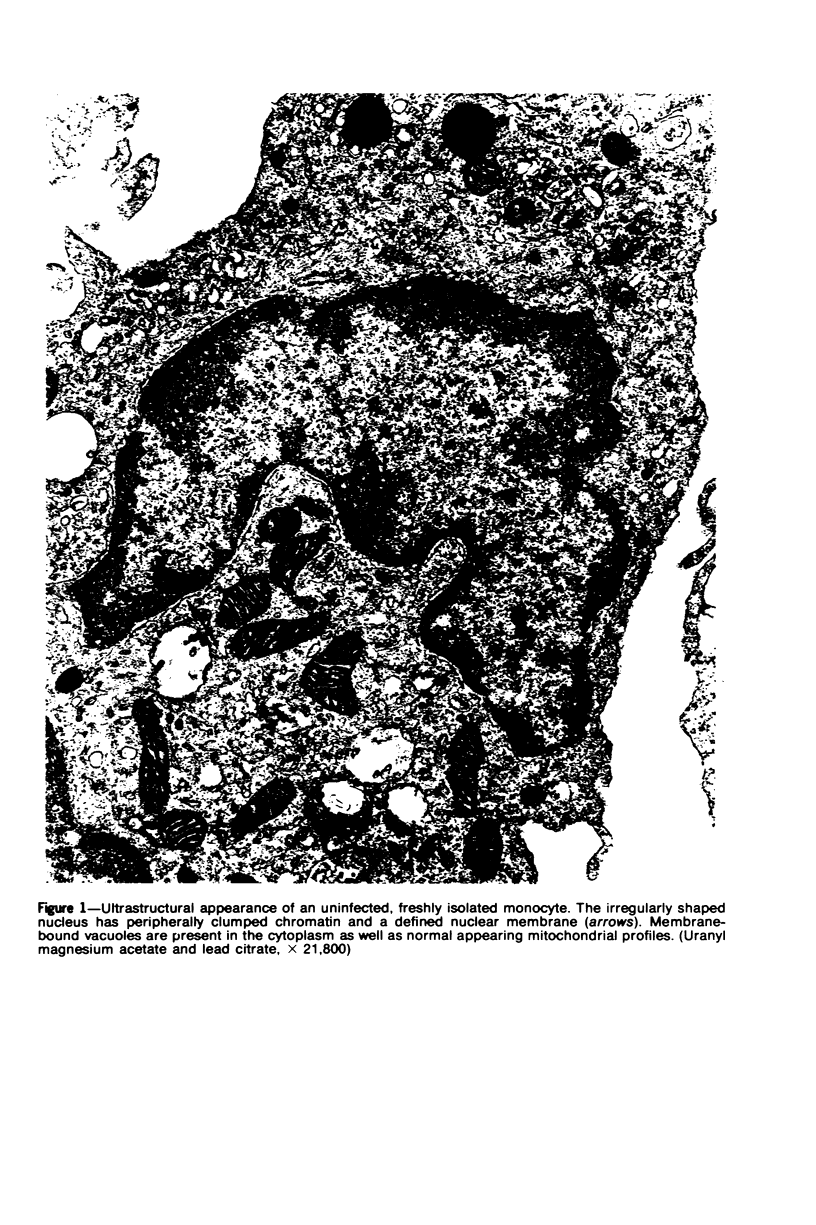

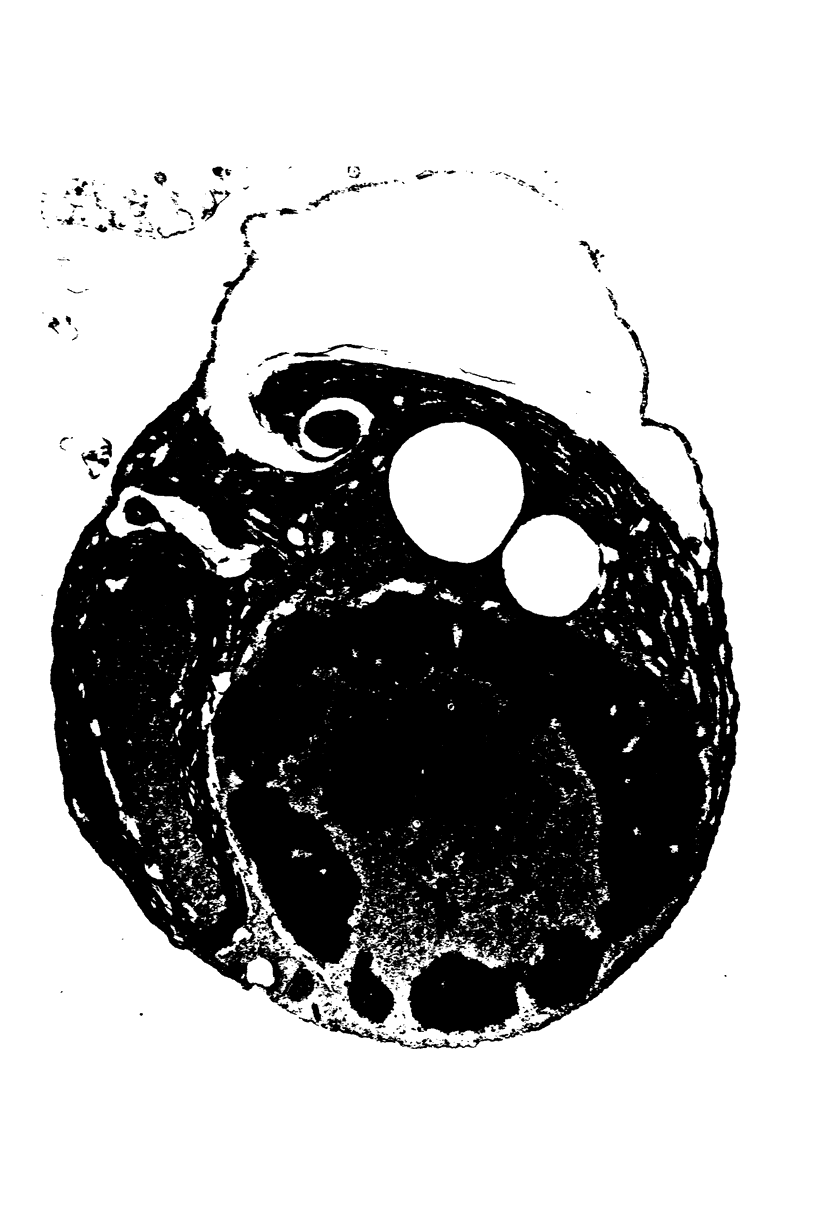
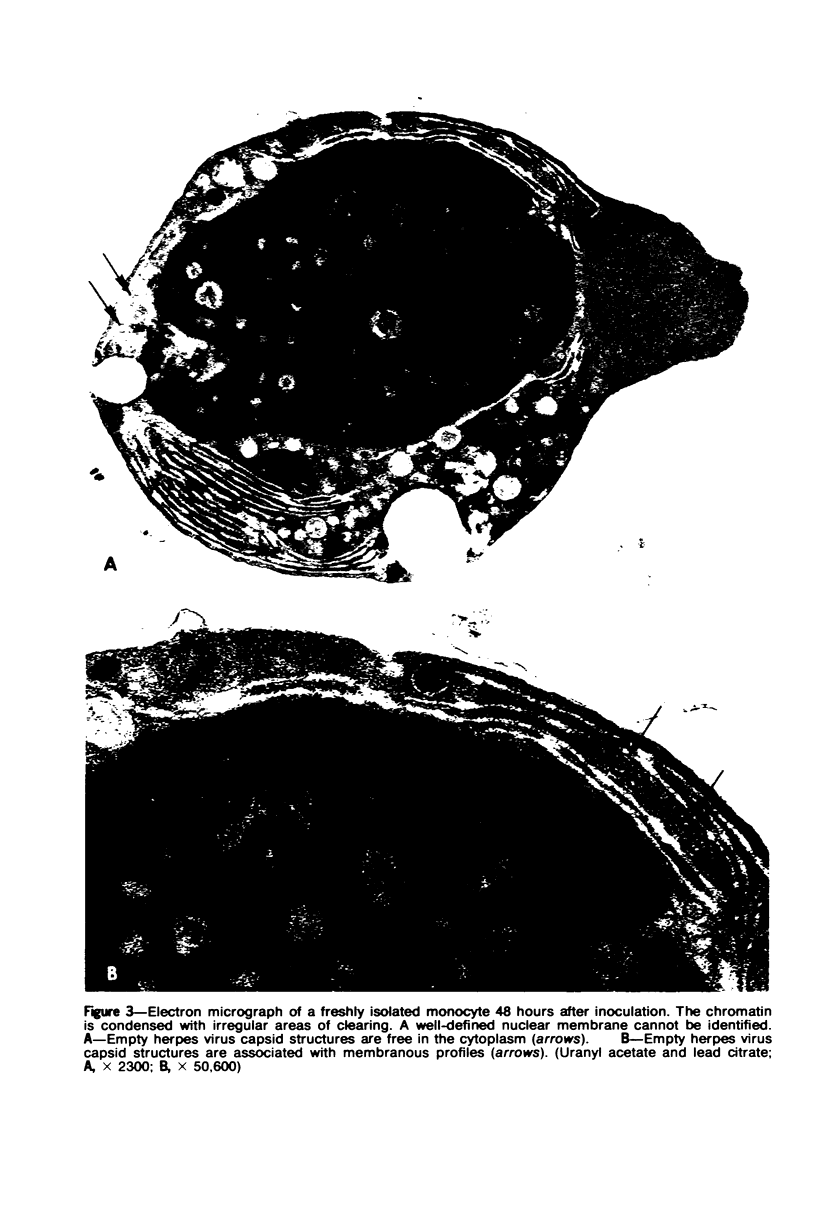
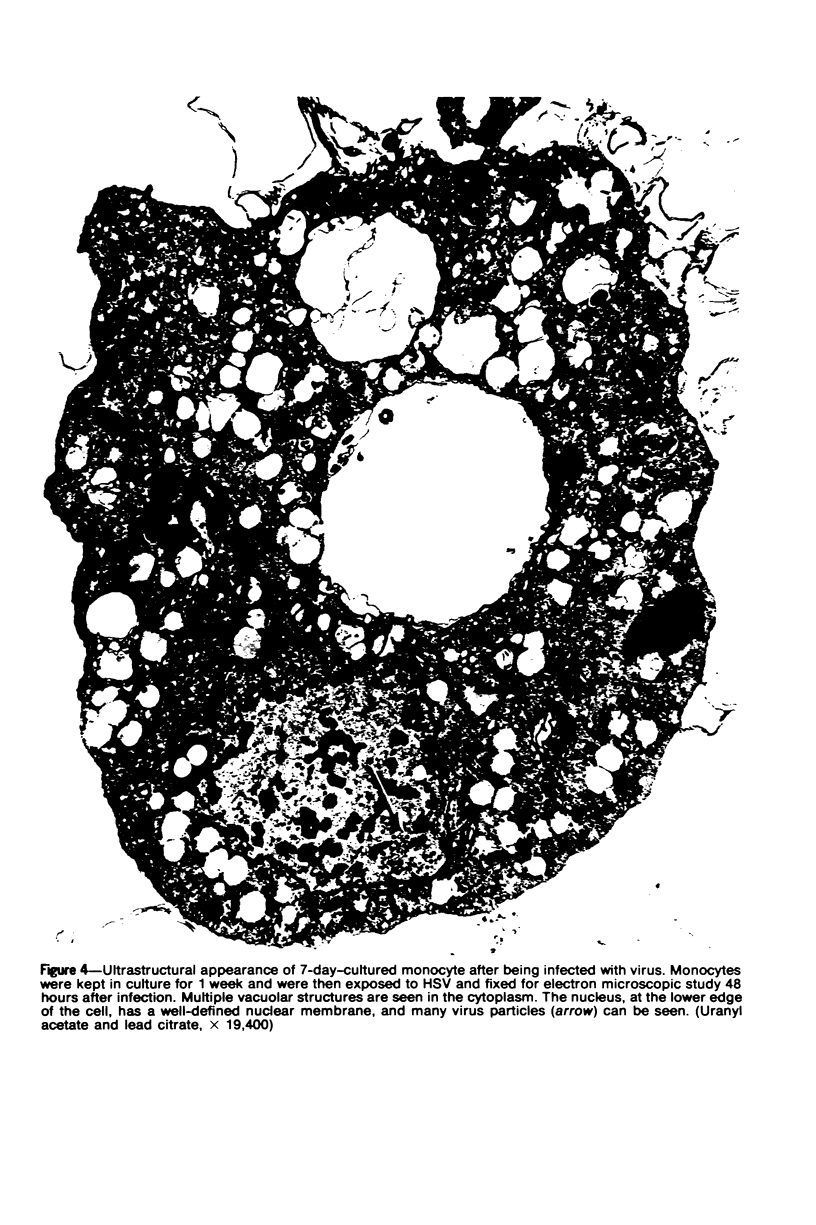

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHE W. K., SCHERP H. W. ANTIGENIC ANALYSIS OF HERPES SIMPLEX VIRUS BY NEUTRALIZATION KINETICS. J Immunol. 1963 Nov;91:658–665. [PubMed] [Google Scholar]
- Allison A. C. On the role of mononuclear phagocytes in immunity against viruses. Prog Med Virol. 1974;18(0):15–31. [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker Y., Olshevsky U., Levitt J. The role of arginine in the replication of herpes simplex virus. J Gen Virol. 1967 Oct;1(4):471–478. doi: 10.1099/0022-1317-1-4-471. [DOI] [PubMed] [Google Scholar]
- Bennett W. E., Cohn Z. A. The isolation and selected properties of blood monocytes. J Exp Med. 1966 Jan 1;123(1):145–160. doi: 10.1084/jem.123.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J. J., Mäntyjärvi R. A., Rapp F. Mechanism of the restricted growth of herpes simplex virus type 2 in a hamster cell line. J Gen Virol. 1972 Sep;16(3):255–264. doi: 10.1099/0022-1317-16-3-255. [DOI] [PubMed] [Google Scholar]
- Fischer D., Van der Weyden M. B., Snyderman R., Kelley W. N. A role for adenosine deaminase in human monocyte maturation. J Clin Invest. 1976 Aug;58(2):399–407. doi: 10.1172/JCI108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Study of the mechanism of innate resistance to virus infection. J Cell Comp Physiol. 1962 Jun;59:333–373. doi: 10.1002/jcp.1030590313. [DOI] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. II. A CELLULAR BASIS FOR THE DEVELOPMENT OF RESISTANCE WITH AGE. J Exp Med. 1964 Sep 1;120:359–374. doi: 10.1084/jem.120.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinerman E. S., Snyderman R., Daniels C. A. Depression of human monocyte chemotaxis by herpes simplex and influenza viruses. J Immunol. 1974 Nov;113(5):1562–1567. [PubMed] [Google Scholar]
- Kohl S., Starr S. E., oleske J. M., Shore S. L., Ashman R. B., Nahmias A. J. Human monocyte-macrophage-mediated antibody-dependent cytotoxicity to herpes simplex virus-infected cells. J Immunol. 1977 Mar;118(3):729–735. [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii S., Rosenkranz H. S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. 3. Effect of hydroxyurea. J Virol. 1968 Oct;2(10):1163–1171. doi: 10.1128/jvi.2.10.1163-1171.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A. Temperature-sensitive mutants of herpesviruses. Curr Top Microbiol Immunol. 1975;70:51–100. doi: 10.1007/978-3-642-66101-3_3. [DOI] [PubMed] [Google Scholar]
- Shinkai K. Plaque morphology of herpes simplex virus in various cells under liquid overlay as a marker for its type differentiation. Jpn J Microbiol. 1975 Dec;19(6):459–462. doi: 10.1111/j.1348-0421.1975.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Spring S. B., Roizman B., Schwartz J. Herpes simplex virus products in productive and abortive infection. II. Electron microscopic and immunological evidence for failure of virus envelopment as a cause of abortive infection. J Virol. 1968 Apr;2(4):384–392. doi: 10.1128/jvi.2.4.384-392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Restriction of herpes simplex virus by macrophages. An analysis of the cell-virus interaction. J Exp Med. 1971 Jan 1;133(1):19–38. doi: 10.1084/jem.133.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G. S., Ballard R. Interaction of mouse peritoneal macrophages with fixed rabies virus in vivo and in vitro. J Gen Virol. 1976 Feb;30(2):223–231. doi: 10.1099/0022-1317-30-2-223. [DOI] [PubMed] [Google Scholar]