Abstract
The individual and combined effects of neonatal thymectomy and whole-body irradiation on the prevalence of benign and malignant tumors in germ-free female mice of the Charles Rivers line were studied to determine if a portion of the tumorigenic effects of irradiation can be attributed to injury of the thymic-dependent component of the immune response. Neonatal thymectomy increased a) the incidence of benign and malignant tumors and b) the prevalence of multiple primary neoplasms in an individual mouse. Whole-body exposure to 700 rad at 6 weeks of age further increased th incidence of tumors, but the relative magnitude of this increase was less pronounced than in sham-operated controls. Thus, the cumulative effects of thymectomy plus irradiation are less pronounced than the sum of the individual effects. One of several possible explanations for this observation is that a portion of the carcinogenic effects of whole-body irradiation is mediated by suppression of the thymic-dependent component of the immune response.
Full text
PDF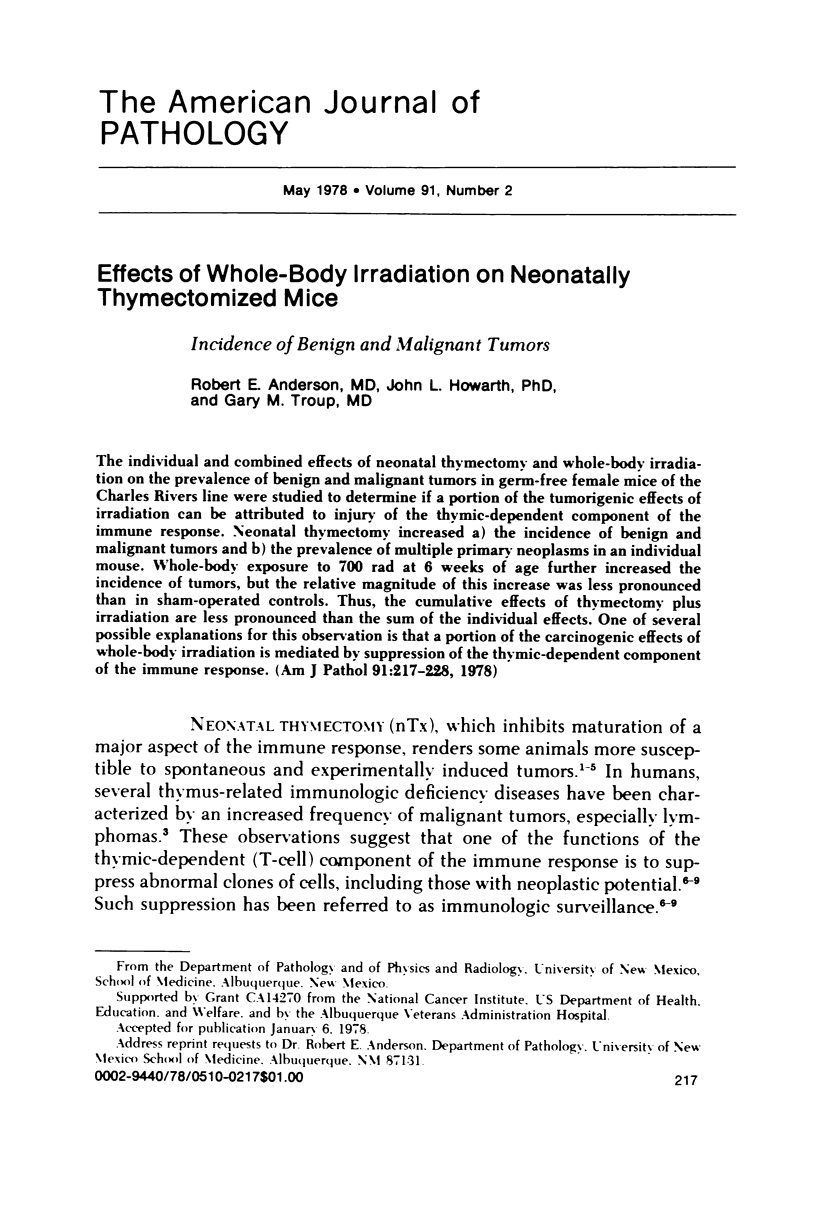







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Doughty W. E., Howarth J. L. Radiation-induced lymphopenia. Recovery in thymectomized and splenectomized germ-free mice. Arch Pathol. 1971 Dec;92(6):480–483. [PubMed] [Google Scholar]
- Anderson R. E., Doughty W. E., Stone R. S., Howarth J. Spontaneous and radiation-related neoplasms in germ-free mice. Arch Pathol. 1972 Sep;94(3):250–254. [PubMed] [Google Scholar]
- Anderson R. E., Howarth J. L., Stone R. S. Acute response of germ-free and conventional mice to ionizing radiation. Arch Pathol. 1968 Dec;86(6):676–680. [PubMed] [Google Scholar]
- Anderson R. E., Scaletti J. V., Howarth J. L. Radiation-induced life shortening in germfree mice. Exp Gerontol. 1972 Oct;7(5):289–301. doi: 10.1016/0531-5565(72)90037-x. [DOI] [PubMed] [Google Scholar]
- Anderson R. E., Warner N. L. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- BURNET M. Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br Med J. 1957 Apr 13;1(5023):841–847. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley P. C., Woody J., Dunkley M., Feldmann M., McKenzie I. Separation of suppressor and killer T cells by surgace phenotype. Nature. 1976 Aug 5;262(5568):495–497. doi: 10.1038/262495a0. [DOI] [PubMed] [Google Scholar]
- Boyse E. A., Miyazawa M., Aoki T., Old L. J. Ly-A and Ly-B: two systems of lymphocyte isoantigens in the mouse. Proc R Soc Lond B Biol Sci. 1968 Jun 11;170(1019):175–193. doi: 10.1098/rspb.1968.0032. [DOI] [PubMed] [Google Scholar]
- Burnet F. M. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- Evans R. L., Breard J. M., Lazarus H., Schlossman S. F., Chess L. Detection, isolation, and functional characterization of two human T-cell subclasses bearing unique differentiation antigens. J Exp Med. 1977 Jan 1;145(1):221–233. doi: 10.1084/jem.145.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Beverley P. C., Dunkley M., Kontiainen S. Different Ly antigen phenotypes of in vitro induced helper and suppressor cells. Nature. 1975 Dec 18;258(5536):614–616. doi: 10.1038/258614a0. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Cohen P., Hencin R., Liebhaber S. A. Suppressor T cells. J Immunol. 1972 Mar;108(3):586–590. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Huber B., Devinsky O., Gershon R. K., Cantor H. Cell-mediated immunity: delayed-type hypersensitivity and cytotoxic responses are mediated by different T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1534–1539. doi: 10.1084/jem.143.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Benacerraf B. Carrier function in anti-hapten antibody responses. VI. Establishment of experimental conditions for either inhibitory or enhancing influences of carrier-specific cells on antibody production. J Immunol. 1973 Jan;110(1):107–117. [PubMed] [Google Scholar]
- Kisielow P., Hirst J. A., Shiku H., Beverley P. C., Hoffman M. K., Boyse E. A., Oettgen H. F. Ly antigens as markers for functionally distinct subpopulations of thymus-derived lymphocytes of the mouse. Nature. 1975 Jan 17;253(5488):219–220. doi: 10.1038/253219a0. [DOI] [PubMed] [Google Scholar]
- Law L. W. Immunologic responsiveness and the induction of experimental neoplasms. Cancer Res. 1966 Jun;26(6):1121–1134. [PubMed] [Google Scholar]
- Law L. W. Studies of thymic function with emphasis on the role of the thymus in oncogenesis. Cancer Res. 1966 Apr;26(4):551–574. [PubMed] [Google Scholar]
- MARTINEZ C. EFFECT OF EARLY THYMECTOMY ON DEVELOPMENT OF MAMMARY TUMOURS IN MICE. Nature. 1964 Sep 12;203:1188–1188. doi: 10.1038/2031188a0. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Cohen P. L. Ontogeny of mouse T-lymphocyte function. Fed Proc. 1975 Feb;34(2):137–140. [PubMed] [Google Scholar]
- Prehn R. T. Perspectives on oncogenesis: does immunity stimulate or inhibit neoplasia? J Reticuloendothel Soc. 1971 Jul;10(1):1–16. [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubinian J. R., Blair P. B. Inhibition of mammary tumors by incomplete T-cell depletion. J Natl Cancer Inst. 1977 Mar;58(3):727–734. doi: 10.1093/jnci/58.3.727. [DOI] [PubMed] [Google Scholar]
- Rygaard J., Povlsen C. O. Is immunological surveillance not a cell-mediated immune function? Transplantation. 1974 Jan 1;17(1):135–136. [PubMed] [Google Scholar]
- Sanford B. H., Kohn H. I., Daly J. J., Soo S. F. Long-term spontaneous tumor incidence in neonatally thymectomized mice. J Immunol. 1973 May;110(5):1437–1439. [PubMed] [Google Scholar]
- Smith C. S., Pilgrim H. I. Spontaneous neoplasms in germfree BALB/cPi mice. Proc Soc Exp Biol Med. 1971 Nov;138(2):542–544. doi: 10.3181/00379727-138-35935. [DOI] [PubMed] [Google Scholar]
- Trainin N., Linker-Israeli M. Increased incidence of spontaneous lung adenomas in mice following neonatal thymectomy. Isr J Med Sci. 1971 Jan;7(1):36–41. [PubMed] [Google Scholar]
- Vadas M. A., Miller J. F., McKenzie I. F., Chism S. E., Shen F. W., Boyse E. A., Gamble J. R., Whitelaw A. M. Ly and Ia antigen phenotypes of T cells involved in delayed-type hypersensitivity and in suppression. J Exp Med. 1976 Jul 1;144(1):10–19. doi: 10.1084/jem.144.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Bealmear M., Sjodin K. A technique for thymectomizing germfree mice. J Appl Physiol. 1966 Jan;21(1):279–281. doi: 10.1152/jappl.1966.21.1.279. [DOI] [PubMed] [Google Scholar]
- Woody J. N., Feldmann M., Beverley P. C., McKenzie I. F. Expression of alloantigens LY-5 and LY-6 on cytotoxic effector cells. J Immunol. 1977 May;118(5):1739–1743. [PubMed] [Google Scholar]
- Yunis E. J., Martinez C., Smith J., Stutman O., Good R. A. Spontaneous mammary adenocarcinoma in mice: influence of thymectomy and reconstitution with thymus grafts or spleen cells. Cancer Res. 1969 Jan;29(1):174–178. [PubMed] [Google Scholar]



