Abstract
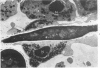
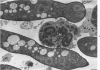
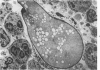
Evidence suggests that neutrophils are important in host defenses against invasive aspergillosis and mucormycosis, although hyphae in these lesions are too large to be phagocytized. Interactions of neutrophils with hyphae of Aspergillus fumigatus and Rhizopus oryzae were studed in vitro. Light and electron microscopic observations indicated that neutrophils attached to and spread over the surfaces of hyphae, even in the absence of serum. This was followed by dramatic morphologic changes which suggested severe damage and probably death of hyphae. An assay of neutrophil-induced reduction of uptake of radioisotopes was used to quantitate damage to the fungi by neutrophils from normal subjects. Damage to hyphae was inhibited by a variety of compounds which are known to affect neutrophil surface functions, motility, and metabolism. Use of inhibitors of oxidative microbicidal mechanisms of neutrophils indicated the central importance of these mechanisms in damage to hyphae. Inhibitors of neutrophil cationic proteins altered damage only to Rhizopus. Damage to hyphae by lysozyme suggested that it may play a secondary role in the neutrophil, primarily against Aspergillus. This new nonphagocytic mechanism may play an important role in host defenses against these and other hyphal forms of fungi.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson E., Wilson D., Arky R. A. Rhinocerebral phycomycosis in association with diabetic ketoacidosis. Report of two cases and a review of clinical and experimental experience with amphotericin B therapy. Ann Intern Med. 1967 Apr;66(4):735–742. doi: 10.7326/0003-4819-66-4-735. [DOI] [PubMed] [Google Scholar]
- Aisner J., Wiernik P. H., Schimpff S. C. Treatment of invasive aspergillosis: relation of early diagnosis and treatment to response. Ann Intern Med. 1977 May;86(5):539–543. doi: 10.7326/0003-4819-86-5-539. [DOI] [PubMed] [Google Scholar]
- Battock D. J., Grausz H., Bobrowsky M., Littman M. L. Alternate-day amphotericin B therapy in the treatment of rhinocerebral phycomycosis (mucormycosis). Ann Intern Med. 1968 Jan;68(1):122–137. doi: 10.7326/0003-4819-68-1-122. [DOI] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- Braf Z. F., Altmann G., Ostfeld E. Fungal infections after renal transplantation. Isr J Med Sci. 1976 Jul;12(7):674–677. [PubMed] [Google Scholar]
- Burton J. R., Zachery J. B., Bessin R., Rathbun H. K., Greenough W. B., 3rd, Sterioff S., Wright J. R., Slavin R. E., Williams G. M. Aspergillosis in four renal transplant recipients. Diagnosis and effective treatment with amphotericin B. Ann Intern Med. 1972 Sep;77(3):383–388. doi: 10.7326/0003-4819-77-3-383. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chang H. Y., Rodriguez V., Narboni G., Bodey G. P., Luna M. A., Freireich E. J. Causes of death in adults with acute leukemia. Medicine (Baltimore) 1976 May;55(3):259–268. doi: 10.1097/00005792-197605000-00005. [DOI] [PubMed] [Google Scholar]
- HUTTER R. V. Phycomycetous infection (mucormycosis) in cancer patients: a complication of therapy. Cancer. 1959 Mar-Apr;12(2):330–350. doi: 10.1002/1097-0142(195903/04)12:2<330::aid-cncr2820120217>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Russell E., Jr, Remington J. S. The compromised host and infection. II. Deep fungal infection. J Infect Dis. 1969 Aug;120(2):169–191. doi: 10.1093/infdis/120.2.169. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Iodination by human polymorphonuclear leukocytes: a re-evaluation. J Lab Clin Med. 1977 Mar;89(3):675–686. [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Jan R. G. Interaction of Aspergillus fumigatus Spores with Human Leukocytes and Serum. Infect Immun. 1970 Apr;1(4):345–350. doi: 10.1128/iai.1.4.345-350.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkow L. P., Epstein S. M., Sidransky H., Verney E., Pardo M. The pathogenesis of experimental pulmonary aspergillosis. An ultrastructural study of alveolar macrophages after phagocytosis of a flavus spores in vivo. Am J Pathol. 1971 Jan;62(1):57–74. [PMC free article] [PubMed] [Google Scholar]
- Meyer R. D., Rosen P., Armstrong D. Phycomycosis complicating leukemia and lymphoma. Ann Intern Med. 1972 Dec;77(6):871–879. doi: 10.7326/0003-4819-77-6-871. [DOI] [PubMed] [Google Scholar]
- Meyer R. D., Young L. S., Armstrong D., Yu B. Aspergillosis complicating neoplastic disease. Am J Med. 1973 Jan;54(1):6–15. doi: 10.1016/0002-9343(73)90077-6. [DOI] [PubMed] [Google Scholar]
- Michl J., Ohlbaum D. J., Silverstein S. C. 2-Deoxyglucose selectively inhibits Fc and complement receptor-mediated phagocytosis in mouse peritoneal macrophages II. Dissociation of the inhibitory effects of 2-deoxyglucose on phagocytosis and ATP generation. J Exp Med. 1976 Dec 1;144(6):1484–1493. doi: 10.1084/jem.144.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickenberg I. D., Root R. K., Wolff S. M. Leukocytic function in hypogammaglobulinemia. J Clin Invest. 1970 Aug;49(8):1528–1538. doi: 10.1172/JCI106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E. Aspergillus pneumonia in hematologic malignancy. Improvements in diagnosis and therapy. Arch Intern Med. 1977 Jun;137(6):769–771. [PubMed] [Google Scholar]
- Querinjean P., Masson P. L., Heremans J. F. Molecular weight, single-chain structure and amino acid composition of human lactoferrin. Eur J Biochem. 1971 Jun 11;20(3):420–425. doi: 10.1111/j.1432-1033.1971.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Raubitschek A. A., Levin A. S., Stites D. P., Shaw E. B., Fudenberg H. H. Normal granulocyte infusion therapy for aspergillosis in chronic granulomatous disease. Pediatrics. 1973 Feb;51(2):230–233. [PubMed] [Google Scholar]
- Rifkind D., Marchioro T. L., Schneck S. A., Hill R. B., Jr Systemic fungal infections complicating renal transplantation and immunosuppressive therapy. Clinical, microbiologic, neurologic and pathologic features. Am J Med. 1967 Jul;43(1):28–38. doi: 10.1016/0002-9343(67)90146-5. [DOI] [PubMed] [Google Scholar]
- Rose H. D., Varkey B. Deep mycotic infection in the hospitalized adult: a study of 123 patients. Medicine (Baltimore) 1975 Nov;54(6):499–507. doi: 10.1097/00005792-197511000-00004. [DOI] [PubMed] [Google Scholar]
- Sickles E. A., Young V. M., Greene W. H., Wiernik P. H. Pneumonia in acute leukemia. Ann Intern Med. 1973 Oct;79(4):528–534. doi: 10.7326/0003-4819-79-4-528. [DOI] [PubMed] [Google Scholar]
- Sidransky H., Epstein S. M., Verney E., Horowitz C. Experimental visceral aspergillosis. Am J Pathol. 1972 Oct;69(1):55–70. [PMC free article] [PubMed] [Google Scholar]
- Sieving R. R., Kauffman C. A., Watanakunakorn C. Deep fungal infection in systemic lupus erythematosus - three cases reported, literature reviewed. J Rheumatol. 1975 Mar;2(1):61–72. [PubMed] [Google Scholar]
- Singer C., Kaplan M. H., Armstrong D. Bacteremia and fungemia complicating neoplastic disease. A study of 364 cases. Am J Med. 1977 May;62(5):731–742. doi: 10.1016/0002-9343(77)90876-2. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Babior B. M. Evidence for hydroxyl radical production by human neutrophils. J Clin Invest. 1977 Aug;60(2):374–379. doi: 10.1172/JCI108786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner K. J., Hackshaw R., Papadimitriou J., Perrott J. The pathogenesis of experimental pulmonary aspergillosis in normal and cortisone-treated rats. J Pathol. 1976 Feb;118(2):65–73. doi: 10.1002/path.1711180202. [DOI] [PubMed] [Google Scholar]
- Turner K. J., Hackshaw R., Papadimitriou J., Wetherall J. D., Perrott J. Experimental aspergillosis in rats infected via intraperitoneal and subcutaneous routes. Immunology. 1975 Jul;29(1):55–66. [PMC free article] [PubMed] [Google Scholar]
- Young R. C., Bennett J. E., Vogel C. L., Carbone P. P., DeVita V. T. Aspergillosis. The spectrum of the disease in 98 patients. Medicine (Baltimore) 1970 Mar;49(2):147–173. doi: 10.1097/00005792-197003000-00002. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Effects of cytochalasin B on polymorphonuclear leucocyte locomotion, phagocytosis and glycolysis. Exp Cell Res. 1972 Aug;73(2):383–393. doi: 10.1016/0014-4827(72)90062-6. [DOI] [PubMed] [Google Scholar]