Abstract
The migration patterns of normal mouse embryo fibroblast (MEF) cells and mouse fibrosarcoma (FS) cells were compared in two assay systems. The two assay systems used were themodified Boyden chamber (micropore membrane) assay and the agarose drop explant assay. In both assays the major population of MEF cells exhibited a greater rate of migration than the major population of FS cells. However, a small subpopulation of FS cells which had a much greater rate of migration than the major population of either MEF or FS cells was detected in the agarose drop assay. A number of drugs which are known to inhibit the migration of leukocytes were tested against the MEF and FS cells. Concentrations were found that inhibited the major population of both groups by greater than 90%. However, at concentrations which inhibited the migration of the major population of FS cells by greater than 90%, a small group of fast-moving cells was still detected. Although the fast-moving cells were relatively resistant to treatment with the various drugs, this group was sensitive to a factor in serum. When normal human serum was used in place of fetal calf serum, the migration of the major population of FS cells was inhibited very little but movement of the fast-moving population was completely eliminated. We speculate that the small subgroup of fast-moving cells may be responsible for the invasive nature of the FS cells.
Full text
PDF

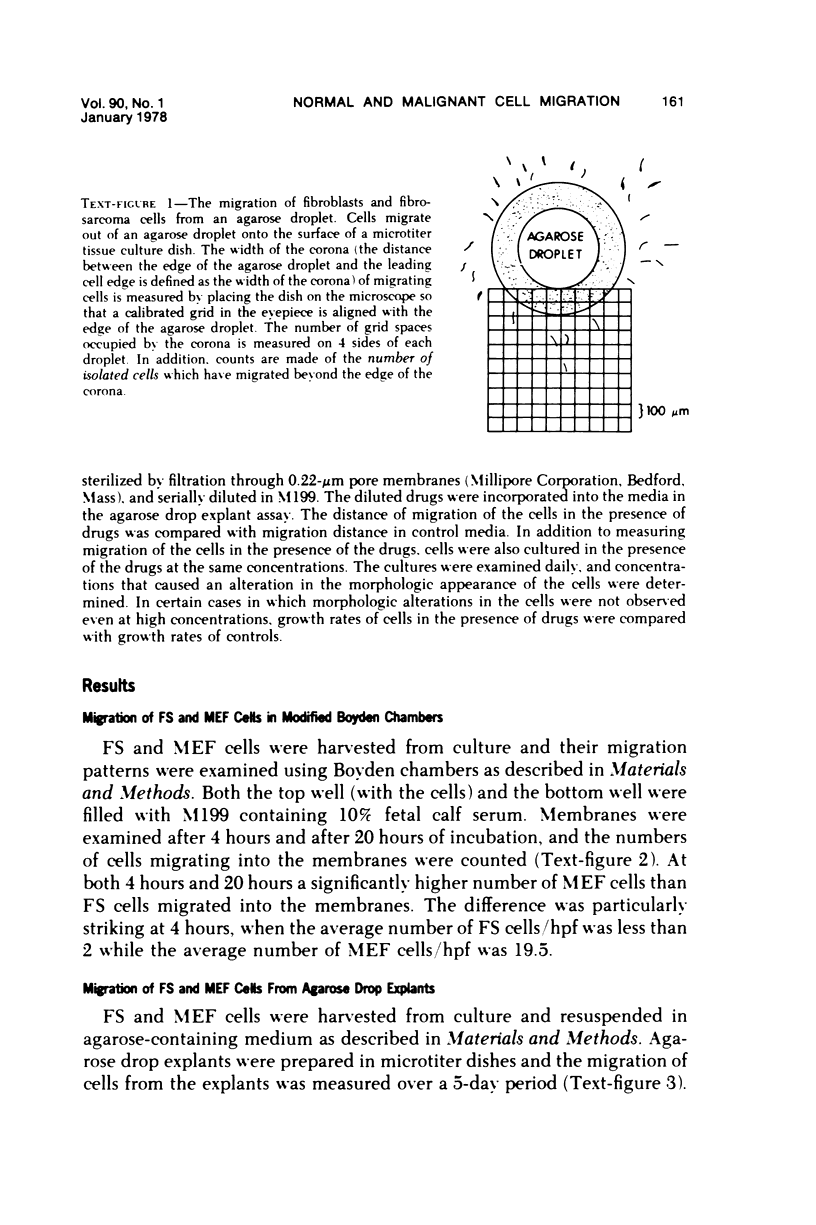
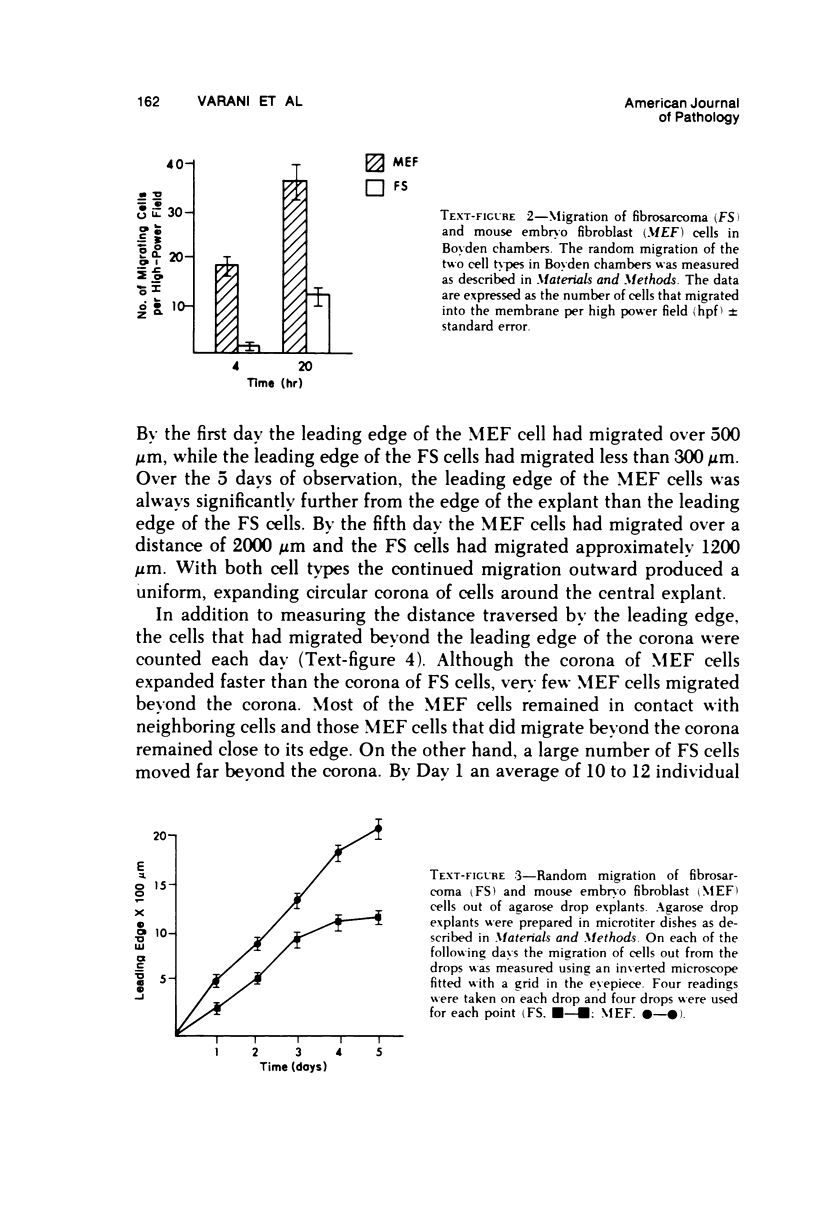
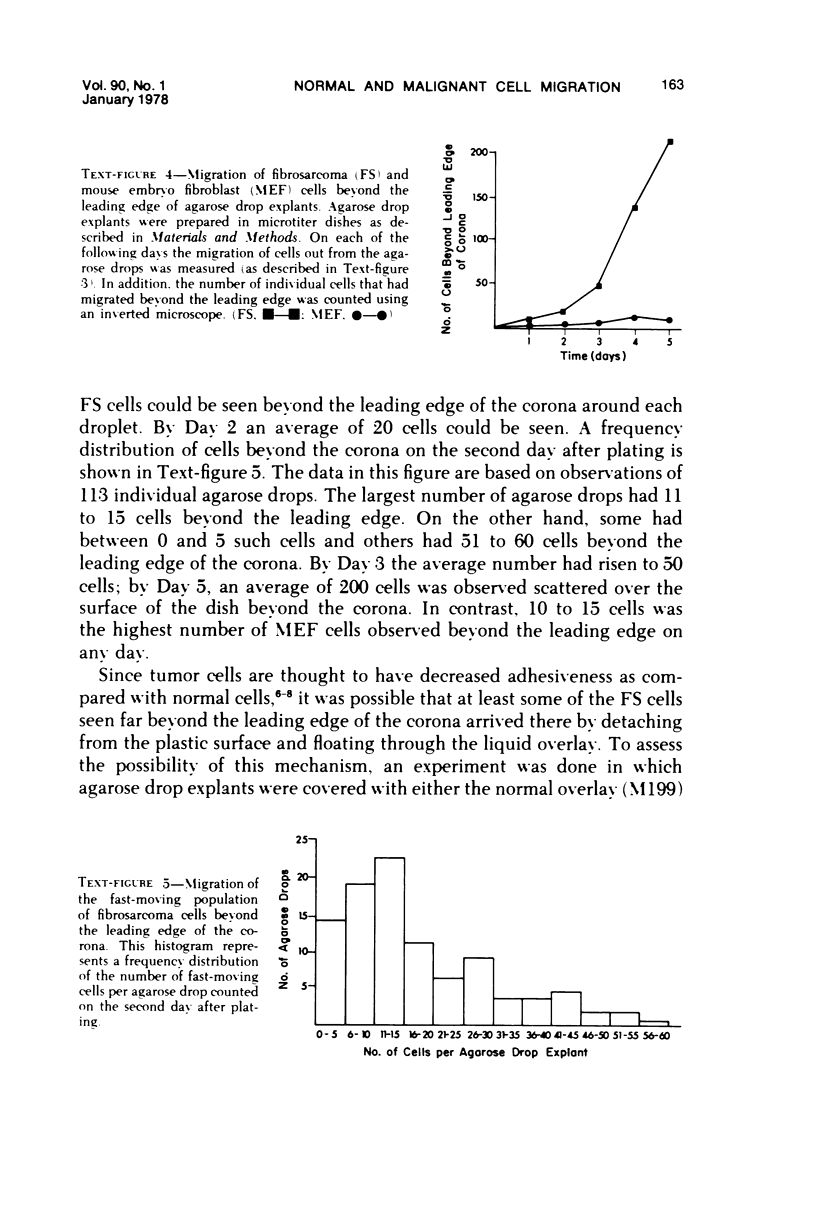


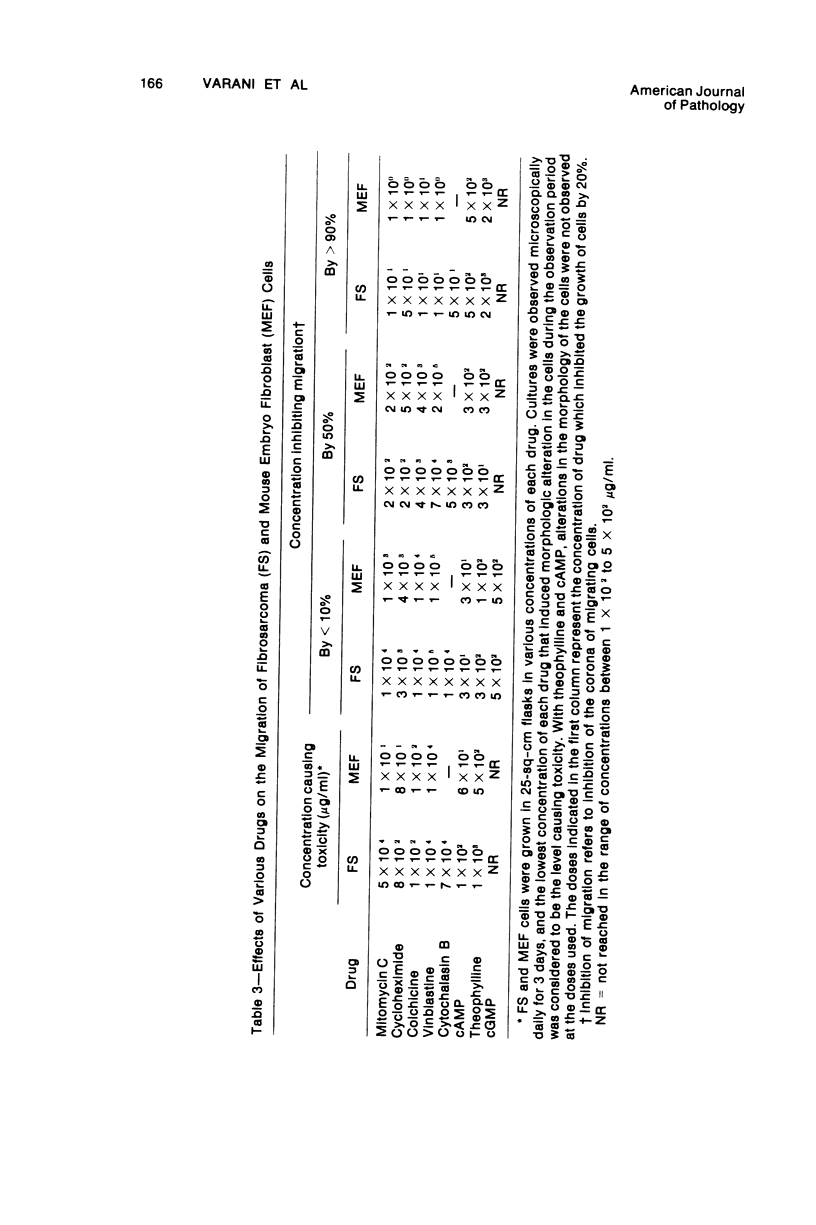





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., AMBROSE E. J. Interference microscope studies of cell contacts in tissue culture. Exp Cell Res. 1958 Oct;15(2):332–345. doi: 10.1016/0014-4827(58)90034-x. [DOI] [PubMed] [Google Scholar]
- ABERCROMBIE M., HEAYSMAN J. E. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res. 1953 Sep;5(1):111–131. doi: 10.1016/0014-4827(53)90098-6. [DOI] [PubMed] [Google Scholar]
- ABERCROMBIE M., HEAYSMAN J. E. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res. 1954 May;6(2):293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- BARSKI G., BELEHRADEK J., Jr ETUDE MICROCIN'EMATOGRAPHIQUE DU M'ECANISME D'INVASION CANC'EREUSE EN CULTURES DE TISSU NORMAL ASSOCI'E AUX CELLULES MALIGNES. Exp Cell Res. 1965 Feb;37:464–480. doi: 10.1016/0014-4827(65)90194-1. [DOI] [PubMed] [Google Scholar]
- Bürk R. R. A factor from a transformed cell line that affects cell migration. Proc Natl Acad Sci U S A. 1973 Feb;70(2):369–372. doi: 10.1073/pnas.70.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARPENTER R. R. IN VITRO STUDIES OF CELLULAR HYPERSENSITIVITY. I. SPECIFIC INHIBITION OF MIGRATION OF CELLS FROM ADJUVANT-IMMUNIZED ANIMALS BY PURIFIED PROTEIN DERIVATIVE AND OTHER PROTEIN ANTIGENS. J Immunol. 1963 Dec;91:803–818. [PubMed] [Google Scholar]
- DARKEN M. A. PUROMYCIN INHIBITION OF PROTEIN SYNTHESIS. Pharmacol Rev. 1964 Sep;16:223–243. [PubMed] [Google Scholar]
- Gail M. H., Boone C. W. Density inhibition of motility in 3T3 fibroblasts and their SV40 transformants. Exp Cell Res. 1971 Jan;64(1):156–162. doi: 10.1016/0014-4827(71)90206-0. [DOI] [PubMed] [Google Scholar]
- Gail M. H., Scher C. D., Boone C. W. Dissociation of cell motility from cell proliferation in BALB-c-3T3 fibroblasts. Exp Cell Res. 1972 Feb;70(2):439–443. doi: 10.1016/0014-4827(72)90158-9. [DOI] [PubMed] [Google Scholar]
- Harrington J. T., Jr, Stastny P. Macrophage migration from an agarose droplet: development of a micromethod for assay of delayed hypersensitivity. J Immunol. 1973 Mar;110(3):752–759. [PubMed] [Google Scholar]
- Lipton A., Klinger I., Paul D., Holley R. W. Migration of mouse 3T3 fibroblasts in response to a serum factor. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2799–2801. doi: 10.1073/pnas.68.11.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E. Colchicine: a common mechanism for its anti-inflammatory and anti-mitotic effects. Arthritis Rheum. 1968 Apr;11(2):191–197. doi: 10.1002/art.1780110210. [DOI] [PubMed] [Google Scholar]
- Pick E., Manheimer S. The mechanism of action of soluble lymphocytic mediators. II. Modification of macrophage migration and migration inhibitory factor action by drugs, enzymes and cationic environment. Cell Immunol. 1974 Mar 30;11(1-3):30–46. doi: 10.1016/0008-8749(74)90004-5. [DOI] [PubMed] [Google Scholar]
- Projan A., Tanneberger S. Some findings on movement and contact of human normal and tumour cells in vitro. Eur J Cancer. 1973 Oct;9(10):703–708. doi: 10.1016/0014-2964(73)90059-5. [DOI] [PubMed] [Google Scholar]
- Rajalakshmi S., Liang H., Sarma D. S., Kisilevsky R., Farber E. Cycloheximide, an inhibitor of peptide chain termination or release in liver in vivo and in vitro. Biochem Biophys Res Commun. 1971 Jan 22;42(2):259–265. doi: 10.1016/0006-291x(71)90096-9. [DOI] [PubMed] [Google Scholar]
- Rivkin I., Rosenblatt J., Becker E. L. The role of cyclic AMP in the chemotactic responsiveness and spontaneous motility of rabbit peritoneal neutrophils. The inhibition of neutrophil movement and the elevation of cyclic AMP levels by catecholamines, prostaglandins, theophylline and cholera toxin. J Immunol. 1975 Oct;115(4):1126–1134. [PubMed] [Google Scholar]
- Romualdez A. G., Jr, Ward P. A. A unique complement derived chemotactic factor for tumor cells. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4128–4132. doi: 10.1073/pnas.72.10.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romualdez A. G., Ward P. A., Torikata T. Relationship between the C5 peptides chemotactic for leukocytes and tumor cells. J Immunol. 1976 Nov;117(5 PT2):1762–1766. [PubMed] [Google Scholar]
- Tomasz M., Mercado C. M., Olson J., Chatterjie N. The mode of interaction of mitomycin C with deoxyribonucleic acid and other polynucleotides in vitro. Biochemistry. 1974 Nov 19;13(24):4878–4887. doi: 10.1021/bi00721a002. [DOI] [PubMed] [Google Scholar]
- Veselý P., Weiss R. A. Cell locomotion and contact inhibition of normal and neoplastic rat cells. Int J Cancer. 1973 Jan 15;11(1):64–76. doi: 10.1002/ijc.2910110108. [DOI] [PubMed] [Google Scholar]
- Yarnell M. M., Schnebli H. P. Release from density-dependent inhibition of growth in the absence of cell locomotion. J Cell Sci. 1974 Oct;16(1):181–188. doi: 10.1242/jcs.16.1.181. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Effects of cytochalasin B on polymorphonuclear leucocyte locomotion, phagocytosis and glycolysis. Exp Cell Res. 1972 Aug;73(2):383–393. doi: 10.1016/0014-4827(72)90062-6. [DOI] [PubMed] [Google Scholar]



