Abstract
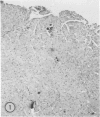
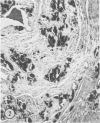
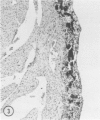
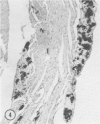
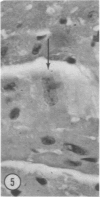
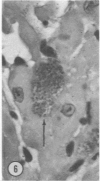
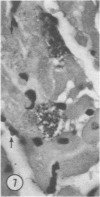
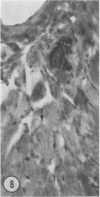
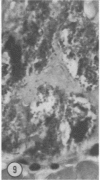
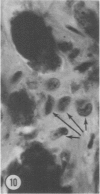
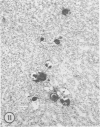
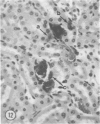
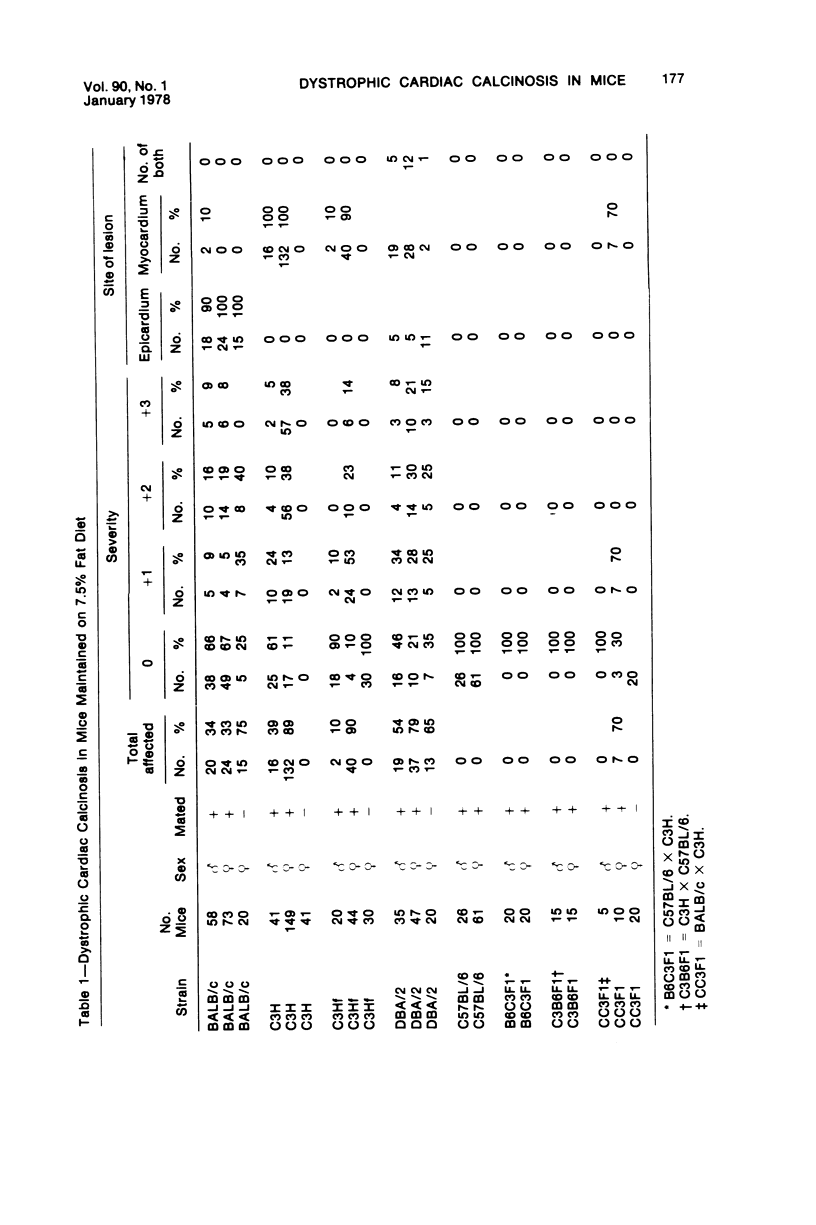
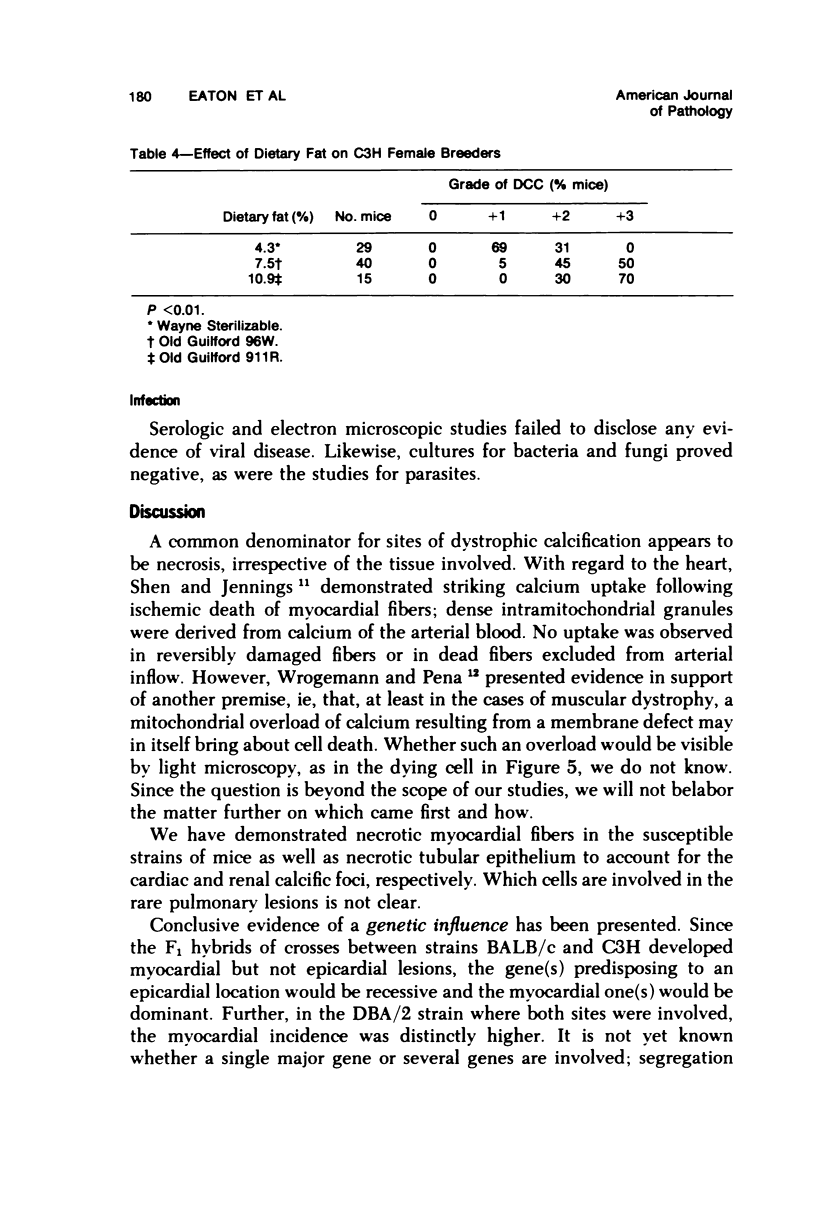
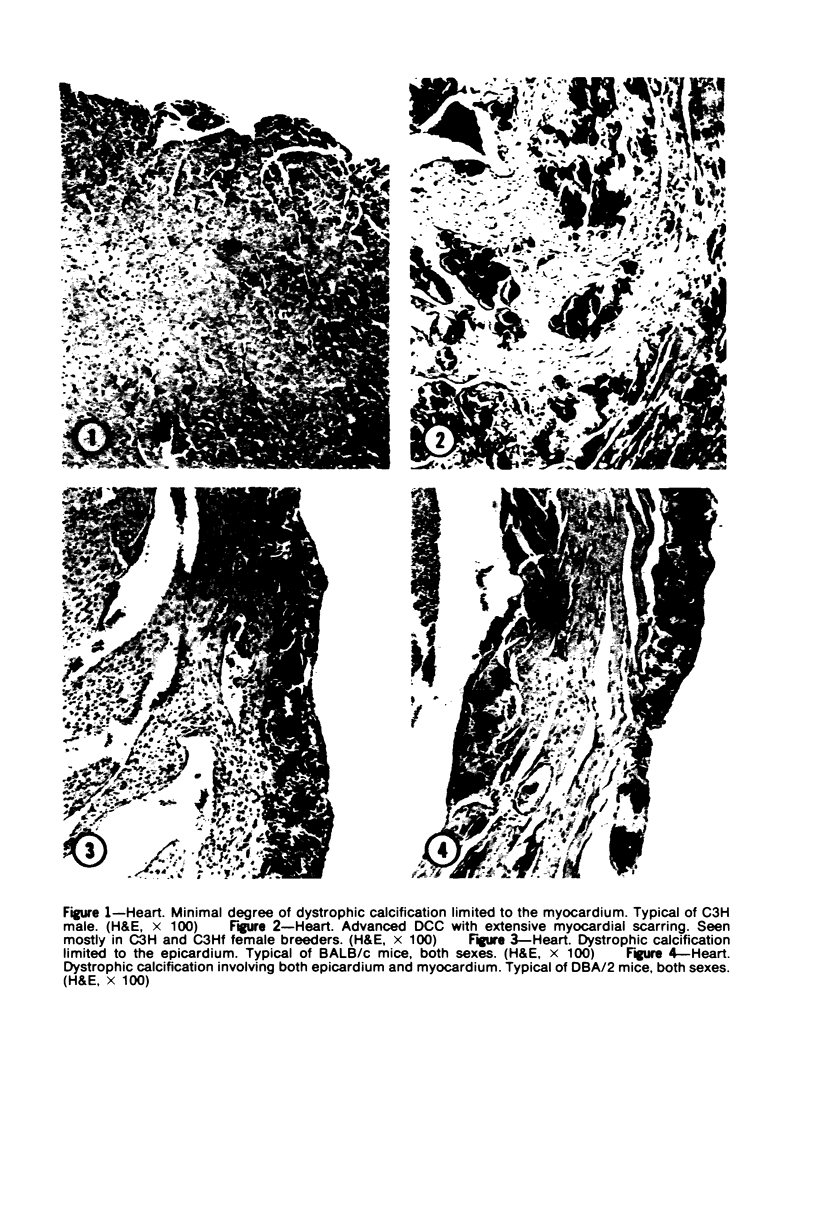
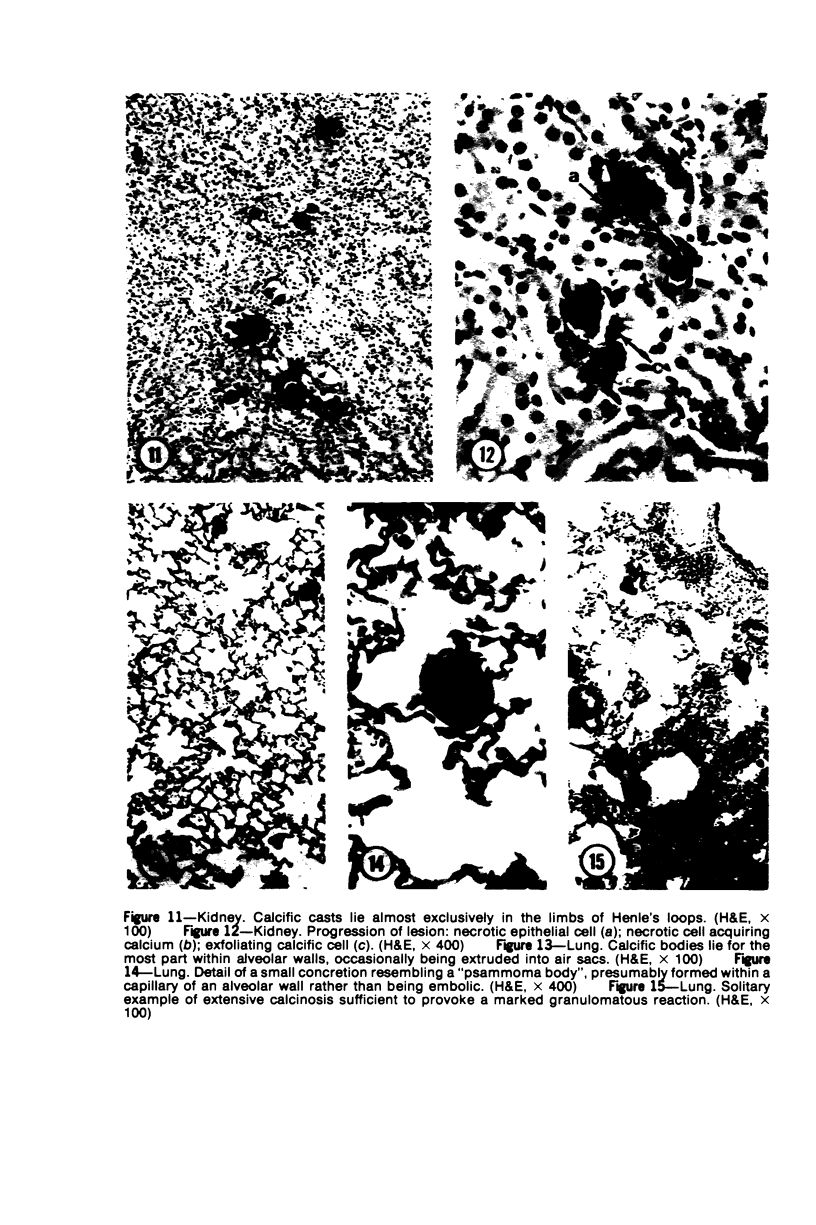
Mice of five inbred strains (BALB/c, C3H, C3Hf, DBA/2, and C57BL/6) of both sexes, mated and virginal, were examined for the incidence, severity, and location of dystrophic cardiac calcinosis (DCC) at various ages. Three hybrids, B6C3F1, C3B6F1, and CC3F1 of both sexes, all mated, were likewise studied. Excepting DBA/2, females of the inbred strains acquired the lesion at a much earlier age than males; DCC appeared in young DBA/s mice of both sexes. DCC in BALB/c mice was almost exclusively epicardial and occurred with equal frequency and severity in mated males and females, with higher incidence but lesser extent in virginal females. The occurrence was highest, the degree most severe, and the location exclusively myocardial in C3H and C3Hf mated females, irrespective of parity, whereas virginal females of these strains were entirely free of disease even after administration of exogenous progesterone. Involvement of males, also myocardial, was relatively minimal, especially in C3Hf mice. Over half the DBA/2 mice were affected, regardless of sex or mating; calcinosis appeared in the epicardium and/or myocardium, predominantly in the myocardium. Strain C57BL/6 was completely devoid of the lesion, as were the two hybrids thereof, B6C3F1 and C3B6F1. The hybrid of BALB/c and C3H showed a high incidence of minimal involvement, exclusively myocardial and limited to breeding females, indicating dominance of the C3H gene(s). Renal calcinosis was uncommon among BALB/c mice but was frequently found in C3H, C3Hf, and DBA/2 strains. Pulmonary calcinosis was rare and limited to C3H and C3Hf female breeders. Mated C3H females fed increasing amounts of fat showed a concomitant rise in incidence and severity of the cardiac lesions. Progression of the lesion from necrotic myocardial fibers to fibrocalcific masses is illustrated, as is formation of the renal deposits.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHBURN A. D., WILLIAMS W. L., COBB F. R. Cardiovascular, hepatic and renal lesions in mice receiving cortisone, estrone and progesterone. Yale J Biol Med. 1963 Feb;35:329–340. [PMC free article] [PubMed] [Google Scholar]
- Ball C. R., Williams W. L. Spontaneous and dietary-induced cardiovascular lesions in DBA mice. Anat Rec. 1965 Jun;152(2):199–209. doi: 10.1002/ar.1091520211. [DOI] [PubMed] [Google Scholar]
- Bellini O., Casazza A. M., di Marco A. Histological and histochemical studies of myocardial lesions in BALBc/Cr mice. Lab Anim Sci. 1976 Apr;26(2 Pt 2):329–333. [PubMed] [Google Scholar]
- Eaton G. J. Intestinal helminths in inbred strains of mice. Lab Anim Sci. 1972 Dec;22(6):850–853. [PubMed] [Google Scholar]
- Frith C. H., Haley T. J., Seymore B. W. Spontaneous epicardial mineralization in BALB/cStCrl mice. Lab Anim Sci. 1975 Dec;25(6):787–787. [PubMed] [Google Scholar]
- LOSTROH A., LI C. H. Deposition induced by hydrocortisone of calcium in the heart tissue of female C3H mice. Nature. 1955 Sep 10;176(4480):504–504. doi: 10.1038/176504a0. [DOI] [PubMed] [Google Scholar]
- Nabors C. E., Ball C. R. Spontaneous calcification in hearts of DBA mice. Anat Rec. 1969 Jun;164(2):153–161. doi: 10.1002/ar.1091640203. [DOI] [PubMed] [Google Scholar]
- Rings R. W., Wagner J. E. Incidence of cardiac and other soft tissue mineralized lesions in DNA-2 mice. Lab Anim Sci. 1972 Jun;22(3):344–352. [PubMed] [Google Scholar]
- SPARKS L. L., ROSENAU W., MACALPIN R. N., DAANE T. A., LI C. H. Production of dystrophic calcification of cardiac muscle in mice by hydrocortisone. Nature. 1955 Sep 10;176(4480):503–504. doi: 10.1038/176503a0. [DOI] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Kinetics of calcium accumulation in acute myocardial ischemic injury. Am J Pathol. 1972 Jun;67(3):441–452. [PMC free article] [PubMed] [Google Scholar]
- Wrogemann K., Pena S. D. Mitochondrial calcium overload: A general mechanism for cell-necrosis in muscle diseases. Lancet. 1976 Mar 27;1(7961):672–674. doi: 10.1016/s0140-6736(76)92781-1. [DOI] [PubMed] [Google Scholar]