Abstract
Nitric oxide (NO) is a potent vasodilator and inhibitor of platelet activation. NO stimulates production of cGMP and activates cGMP-dependent protein kinase (G kinase), which by an unknown mechanism leads to inhibition of Gαq-phospholipase C-inositol 1,4,5-triphosphate signaling and intracellular calcium mobilization for several important agonists, including thromboxane A2 (TXA2). To explore the mechanism of platelet inhibition by NO, activation of platelet TXA2 receptors in the presence of cGMP was studied. The nonhydrolyzable analog 8-bromo-cyclic GMP (8-Br-cGMP) potently inhibited activation of the TXA2-specific GTPase in platelet membranes in a concentration-dependent fashion, suggesting that G kinase catalyzes the phosphorylation of some proximal component of the receptor–G protein signaling pathway. Nanomolar concentrations of G kinase were found to catalyze the phosphorylation of platelet TXA2 receptors in vitro, but not Gαq copurifying with the TXA2 receptors in these experiments. Using immunoaffinity methods, in vivo phosphorylation of TXA2 receptors by cyclic GMP was demonstrated from 32P-labeled cells treated with 8-Br-cGMP. Peptide mapping studies of in vivo phosphorylated TXA2 receptors demonstrated cGMP mediates phosphorylation of the carboxyl terminus of the TXA2 receptor. G kinase also catalyzed the phosphorylation of peptides corresponding to the cytoplasmic tails of both α and β forms of the receptor but not control peptide or a peptide corresponding to the third intracytoplasmic loop of the TXA2 receptor. These data identify TXA2 receptors as cGMP-dependent protein kinase substrates and support a novel mechanism for the inhibition of cell function by NO in which activation of G kinase inhibits signaling by G protein-coupled receptors by catalyzing their phosphorylation.
The vascular endothelium secretes nitric oxide (NO), the most important known endogenous vasodilator (1), which further protects the vessel wall by inhibiting platelet aggregation (2–5), secretion (6), adhesion (7), and fibrinogen binding to its integrin platelet membrane receptor, GpIIbIIIa (8). In both vascular smooth muscle cells and platelets, these effects of NO are known to be mediated by cGMP, which inhibits phospholipase C activation, inositol 1,4,5-triphosphate (InsP3) generation, and [Ca2+]i mobilization (reviewed in ref. 9; see also refs. 8, 10–15). The inhibitory effects of cGMP are principally mediated by cGMP-dependent protein kinase (G kinase) (9, 15–17), but the site(s) at which G kinase acts to inhibit platelet activation is unknown.
The arachidonic acid metabolite thromboxane A2 (TXA2) is a potent stimulus of platelet aggregation and a physiologic regulator of vascular smooth muscle cell contraction (18–20). Inhibitors of TXA2 production decrease ischemic events in clinical populations (21), supporting an important role for TXA2 in in vivo regulation of hemostasis and thrombosis. The signal transduction events initiated by TXA2 stimulation of platelets are well characterized. Two splice variants of the TXA2 receptor have been identified that differ only in their carboxyl termini; the α isoform has a 15-amino acid carboxyl-terminal extension, whereas the β isoform has a 79-amino acid extension (22, 23). Both isoforms are expressed in platelets and cause platelet activation by increasing phosphoinositide-specific phospholipase C (PLC) activity (23, 24) via the pertussis-insensitive GTP binding protein, Gαq (25–32). PLC generates InsP3, which in turn leads to release of intracellular calcium and activation of Ca2+/calmodulin-regulated proteins such as myosin light chain kinase. Myosin light chain kinase then phosphorylates myosin light chain, promoting platelet aggregation and granule secretion (reviewed in ref. 33).
The coupling of seven transmembrane receptors such as the TXA2 receptor to G proteins occurs principally through cytoplasmic receptor domains, especially the third intracellular loop and the cytoplasmic carboxyl-terminal tail (reviewed in refs. 34–36). The receptor–G protein interaction can be uncoupled by receptor phosphorylation, the principal mechanism by which this family of receptors is rapidly desensitized following agonist activation (36). Agonist activation of G protein-coupled receptors is tightly and reciprocally regulated by two types of kinases: G protein receptor kinases (GRK) and second messenger kinases (36). GRK, such as rhodopsin kinase and the β-adrenergic receptor kinases, desensitize receptors only after agonist stimulation, which initiates GRK recruitment and receptor phosphorylation. Cyclic AMP-dependent protein kinase and protein kinase C also have been reported to catalyze the phosphorylation and promote the desensitization of the β-adrenergic and other receptors following agonist activation in vitro (reviewed in ref. 34). However, regulation of G protein-coupled receptors has not been shown previously to involve G kinase, the principal mediator of NO-cGMP signaling. In this report, we demonstrate that cGMP prevents TXA2 receptors from coupling to and activating their cognate G proteins and show further that the TXA2 receptors themselves are substrates for G kinase in vivo. We propose that NO-mediated inhibition of platelet activation may be due in part to phosphorylation of G protein-coupled receptors by G kinase, which disrupts receptor-G protein coupling and thus downstream signaling.
METHODS
GTPase Assay.
TXA2-stimulated GTPase activity was measured in platelet membranes as reported previously (29). In brief, reactions were initiated by the addition of 20–45 μg of platelet membranes to tubes in the absence or presence of 10 μM of the TXA2 analog U46619. The final reaction mixture (total volume = 100 μl) contained 0.4 μM GTP, including 0.6–0.9 μCi of [γ-32P]GTP (1.5–2 × 106 dpm), as well as 100 mM NaCl, 0.1 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol, 0.1 mM ATP, 5 mM phosphocreatine, 100 units/ml creatine phosphokinase, 0.2% bovine serum albumin, 50 mM triethanolamine HCl, pH 7.4, and indicated concentrations of agonist. For experiments with 8-bromo-cyclic GMP (8-Br-cGMP), platelet membranes were incubated at room temperature for 10 min in 8-Br-cGMP (1 nM to 100 μM) prior to stimulation with U46619. GTPase reactions were terminated with 5% activated charcoal/20 mM phosphoric acid, and high affinity GTPase activity was measured and calculated as described (27, 29).
Antibody Production and Immunoaffinity Purification of the TXA2 Receptors.
An antibody to the TXA2 receptor was raised in rabbits using a GST-fusion peptide based on the third cytoplasmic loop of the TXA2 receptor (amino acids 221-TLCHVYHGQEAAQQRPRDSEVEMMAQ-246) (22). The GST-fusion protein was generated by in-frame ligation of the cDNA corresponding to the third intracytoplasmic domain of the receptor to the expression plasmid pGEX3X, as reported previously (37). Antisera were screened by dot-blotting methods using the GST-peptide antigen, with GST alone used as control (38). Peptide antisera TXR2 specifically recognized the intracytoplasmic loop 3 peptide and also recognized a 48–53-kDa protein in human platelets corresponding to the endogenous TXA2 receptor (see Fig. 2). Platelets were prepared from plateletpheresis units as described (29, 37). Platelet lysis conditions with varying detergent and ionic conditions were optimized to maximize recovery of the TXA2 receptors. The radioimmunoprecipitation buffer ultimately chosen for all experiments consisted of 25 mM Tris⋅HCl, pH 7.8, 5 mM MgC12, 2.5 mM EDTA, 0.5% digitonin, with a mixture of protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 100 ng/ml chymostatin, 2 ng/ml aprotinin, 1 μg/ml E-64, 0.5 μg/ml leupeptin, 2.5 μg/ml antipain, 100 μM benzamidine). Platelet lysates were precleared with protein A beads prior to receptor isolation experiments to remove any potential residual human IgG. For isolation of platelet TXA2 receptors, the TXR2 antibody was first purified and concentrated on protein A beads, eluted with 100 mM glycine, pH 3.0, adjusted to pH 7.0, and then covalently linked to Sulfolink coupling gel beads (Pierce). Antibody beads were next washed, mixed with 2 ml of concentrated platelet lysate (from ≈1010 platelets), incubated for 3 hrs with rocking, and then washed extensively with radioimmunoprecipitation buffer. Receptor was next eluted with 100 mM glycine buffer, pH 3.0, as described (38). All immunoblotting studies were performed as described previously and developed using the enhanced chemiluminescence system (Amersham) (39). In some experiments, membranes were stripped with 100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris⋅HCl, pH 6.8, at 50°C for 30 min, blocked, and reprobed with a different antibody. The anti-G kinase antibody RIFB was immunopurified and has been described in detail (40). Anti-Gq/G11 antibodies were the kind gift of D. Manning (University of Pennsylvania) (41).
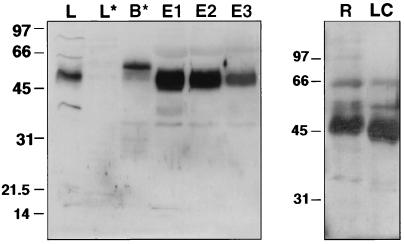
Figure 2.
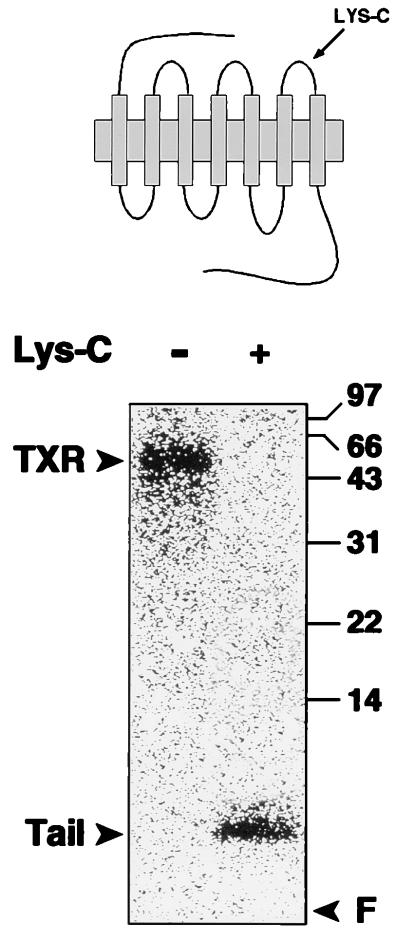
Immunoblot demonstrating immunoaffinity isolation of thromboxane receptors: (Left) Western blot demonstrating isolation of TXA2 receptors from human platelet lysates. TXA2 receptors were isolated by incubating platelet lysates with TXR2 anti-receptor antibody precoupled to agarose beads. Bead eluates (E1–E3) were resolved by SDS/PAGE, transferred to nitrocellulose, and then immunoblotted with TXR2 antibody. L, platelet lysate; L*, lysate after incubating with TXR2 antibody beads; B*, IgG from TXR2 beads; E1–E3, sequential eluates from the TXR2 antibody beads incubated with platelet lysate, washed, and then eluted with low pH glycine buffer. The 47–53-kDa band in lanes L, E1, E2, and E3 is the platelet TXA2 receptor. (Right) Separate experiments demonstrating the expected (ref. 42) small decrease in Mr of the TXA2 receptor following endoproteinase Lys-C digestion. One of four similar experiments. R, TXA2 receptors; LC, receptor digested with endoproteinase Lys-C.
Endoproteinase Lys-C Digestion of the TXA2 Receptor.
Enzymatic proteolysis of the unique lysine residue of the TXA2 receptor (Lys-288) with endoproteinase Lys-C was performed by the method of True and Mais (42) using enzyme conditions recommended by the manufacturer (Boehringer Mannheim). Recombinant N-glucosidase F used for some studies was also from Boehringer Mannheim.
In Vitro Phosphorylation of TXA2 Receptors and of GST-Fusion Peptides.
In vitro phosphorylation experiments with immunopurified TXA2 receptors were performed as described (43, 44). Reactions were in 50 mM Tris⋅HCl, pH 7.5, 5 mM MgCl2, 0.1 mM cGMP for 10–15 min at room temperature and were initiated by addition of 10 μCi of [γ-32P]ATP (6,000 Ci/mmol; Dupont/NEN) and stopped by addition of HCl to a final concentration of 10 mM (43). GST-fusion peptides were prepared from the full-length TXA2 receptor cDNA as template in PCR reactions in which cDNAs corresponding to the third intracytoplasmic loop and the cytoplasmic tails of the TXA2 receptors were amplified. The sequences amplified for each construct corresponds to the third intracytoplasmic loop (iL3) amino acids 220–246; for the TXA2 receptor α C terminus, amino acids 310–343; and for the TXA2 receptor β C terminus, amino acids 310–369. All cDNA were subcloned into the GST-fusion protein expression vector pGEX3X, verified by sequencing, and expressed in Escherichia coli, as above. Purified fusion proteins were introduced into a kinase reaction mixture containing 0.1 mM [γ-32P]ATP and nanomolar concentrations of purified G kinase (see Results). For all in vitro phosphorylation studies, G kinase was prepared and characterized as reported (43).
In Vivo Phosphorylation of TXA2 Receptors.
To examine TXA2 phosphorylation in vivo, HEL cells were chosen for their high density of functional TXA2 receptors identical to the platelet receptor (45, 46) and were labeled with 32P as described (39) and then exposed to buffer alone (10 min), U46619 (5 μM, 10 min), or 8-Br-cGMP (10 mM, 15 min). Reactions were terminated by addition of ice-cold TEB buffer (20 mM Tris⋅Cl, pH 7.4, 5 mM EGTA, 0.128 mg/ml phenylmethylsulfonyl fluoride, 0.15 mg/ml benzamidine, Cl) followed by preparation of solubilized TXA2 receptor fractions in CHAPS buffer (20 mM Tris⋅Cl, pH 7.4, 5 mM EGTA, 25% glycerol, 0.128 mg/ml phenylmethylsulfonyl fluoride, 0.156 mg/ml benzamidine Cl, 10 mM CHAPS) as described in detail previously (47). TXA2 receptor fractions were then purified on and eluted from TXA2 antibody affinity beads as above, resolved by SDS/PAGE, and transferred to nitrocellulose. Receptor was quantified by immunoblotting. and phosphorylation was quantitated by PhosphoImager analysis (39). For peptide mapping of in vivo phosphorylated TXA2 receptors with endoproteinase Lys-C, receptor bands were cut from nitrocellulose and eluted overnight in elution buffer (50 mM Tris⋅Cl, pH 9.0, 2% SDS and 1% Triton X-100) (48). Eluates were then dialyzed for 48 h against 25 mM Tris⋅Cl, pH 8.5, 1 mM EDTA, subjected to enzymatic proteolysis as above, and resolved on 15% SDS/PAGE gels.
RESULTS
cGMP Inhibits U46619-Stimulated GTPase Activation.
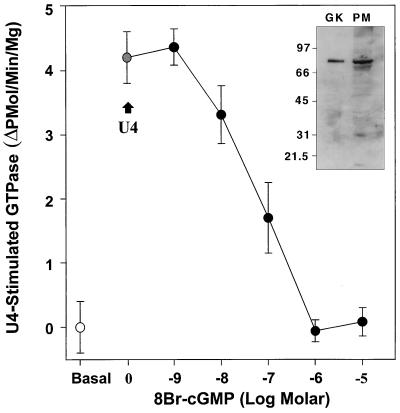
Treatment of platelets with NO or cGMP inhibits the ability of several agonists to stimulate phospholipase C, generate InsP3, and mobilize intracellular calcium ([Ca2+]i) (8, 11, 12, 16, 33). To test whether cGMP can directly influence receptor-G protein coupling, the effect of the nonhydrolyzable cGMP derivative, 8-Br-cGMP, on the TXA2-specific GTPase in human platelet membranes was studied. Membranes were preincubated without or with varying concentrations of 8-Br-cGMP for 10 min and then stimulated with the TXA2 agonist U46619. In untreated membranes, U46619 caused the expected stimulation of TXA2-specific GTPase activity (Fig. 1) (cf. refs. 27, 49), with an average increase in GTPase activity of 5.5 ± 1.3 pmol of 32P/min/mg platelet protein (n = three experiments in triplicate). The effects of cGMP on this TXA2-specific GTPase were studied next by pretreating the platelet membranes with the nonhydrolyzable cGMP analog, 8-Br-cGMP. Pretreatment of the platelet membranes with 8-Br-cGMP inhibited the U46619-stimulated GTPase activity in a dose-dependent fashion (closed circles; mean IC50 = 75 nM; n = 3). In separate studies, pretreatment of platelet membranes with 500 μM 8-Br-cGMP only minimally decreased basal platelet membrane GTPase activity (by 8 ± 9%, n = 4) (data not shown). Immunoblotting studies of the platelet membranes used in these studies demonstrated high levels of G kinase (Fig. 1, inset). In addition, the half-maximal concentration of 8-Br-cGMP necessary for GTPase inhibition in these studies was very similar to the KAct for G kinase (50). Therefore, potential G kinase substrates in the receptor–G protein signaling complex that might explain the observed uncoupling of receptor GTPase activation were explored.
Figure 1.
TXA2 receptor-coupled GTPase activation in human platelet membranes is inhibited by 8-Br-cGMP. Platelet membranes were stimulated with 10 μM of the TXA2 analog U46619 in the absence or presence of varying concentrations of 8-Br-cGMP. Basal GTPase activity (open circle) and U46619-stimulated GTPase activity in buffer-treated membranes (grey circle; +U4) are shown, as is the progressive inhibition of U46619-stimulated GTPase by 8-Br-cGMP (dark circles). The IC50 for inhibition of the TXA2-stimulated GTPase by 8-Br-cGMP is 75 nM (n = three experiments in triplicate). (Inset) Immunoblot demonstrating the presence of high levels of G kinase in the platelet membranes used for these studies. Lane 1, 0.2 μg of purified G kinase; lane 2, 79 μg of total platelet membrane protein.
In Vitro and in Vivo Phosphorylation of the TXA2 Receptors by G Kinase.
To determine whether the TXA2 receptors themselves are substrates for G kinase, an antibody (TXR2) was raised against the third cytoplasmic loop of the TXA2 receptor (see Methods). TXR2 antibody recognized a 48–53-kDa protein in lysates of human platelets (Fig. 2 Left). In previous reports, the TXA2 receptor has been found to migrate as a 48–55-kDa protein on SDS/PAGE systems (42, 51), which overlaps with the Mr of IgG heavy chain. To isolate TXA2 receptors free from the TXR2 antibody IgG heavy chain, TXR2 antibody was covalently coupled to agarose beads and used to immunopurify native TXA2 receptor from concentrated human platelet lysates (Western blot, Fig. 2 Left). The eluted receptor migrated with an Mr of 48–53 kDa, was recognized by the TXR2 antibody on immunoblots, and migrated just below and entirely free from any IgG heavy chain (Fig. 2). The thromboxane receptor contains a single endopeptidase Lys-C cleavage site (Lys-288). Endoproteinase Lys-C digestion of the immunopurified receptor protein led to the expected small decrease in receptor Mr due to release of the receptor C-terminal domain (42) (Fig. 2 Right). Digestion of the receptor with N-glycosidase F also gave the expected decrease in receptor Mr due to deglycosylation, as described previously (42, 51) (data not shown). In separate immunoprecipitation experiments, we also examined whether Gαq/Gα11 or its effector, PLCβ, were phosphorylated in response to NO or cGMP. Immunoprecipitation of Gαq/Gα11 from 32P-labeled platelets treated with buffer alone, the S-nitrosothiol, S-nitroso-N-acetylcysteine (8), or 8-Br-cGMP did not demonstrate any significant G protein phosphorylation (cf. ref. 9). Similarly, in studies using a variety of different monoclonal and polyclonal PLCβ antibodies, no increase in phosphorylation of PLC due to NO or cGMP was detected (M.E.M., unpublished results).
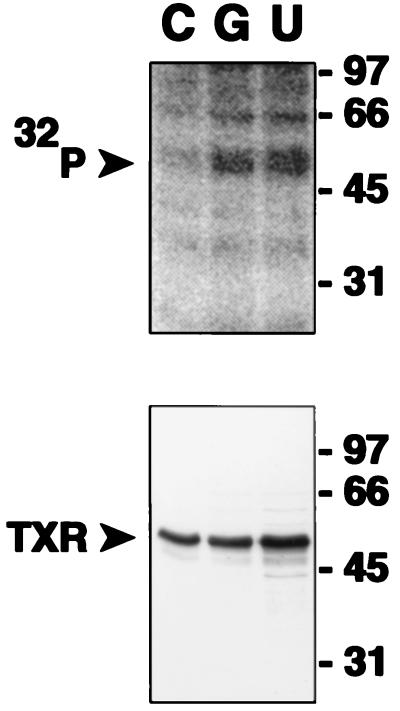
TXA2 receptor eluates also were introduced into kinase reactions with purified G kinase (29–145 nM), which demonstrated in vitro phosphorylation of TXA2 receptors (data not shown; cf. Fig. 5). Interestingly, and as observed previously (26), Gq/G11 G proteins copurified with the TXA2 receptors in these experiments but were not phosphorylated in any of the in vitro G kinase reactions (data not shown). To examine whether cGMP can lead to phosphorylation of TXA2 receptors in vivo, studies were performed using HEL cells, which contain high densities of the platelet TXA2 receptor (45, 46). As expected, TXA2 receptors in these experiments were phosphorylated following treatment of 32P-labeled cells with the thromboxane analog U46619 due to agonist-mediated activation of G protein receptor kinase(s) (36) (Fig. 3, lane U). 8-Br-cGMP treatment also led to clear and significant increases in TXA2 receptor phosphorylation to a level comparable with agonist-induced phosphorylation in five such in vivo experiments (Fig. 3, lane G).
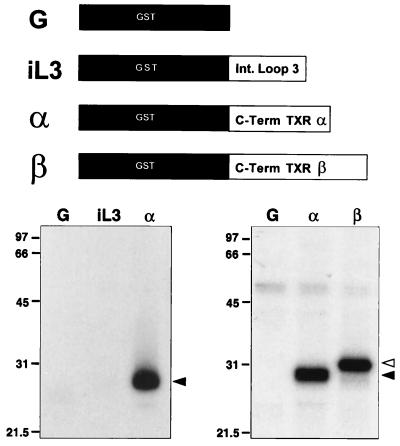
Figure 5.
In vitro phosphorylation of peptide based on the C terminus of TXA2 receptors by G kinase. Kinase reactions contained 8 nM purified G kinase, [γ-32P]ATP, and recombinant GST protein (G), the GST-TXA2 receptor third intracytoplasmic loop (iL3), or the GST-TXA2 receptor tail sequence fusion protein corresponding to the α or β isoforms of the TXA2 receptor (α and β, respectively). Proteins were quantitated by Bradford assay and on Coomassie-stained gels, and the same input concentrations of protein were used for the various peptides. Left and right panels represent two different experiments. Reactions were resolved on 10% SDS/PAGE gels to generate the autoradiographs shown. (Right) Dark arrowhead denotes the TXA2 α carboxyl-terminal fusion protein; open arrowhead denotes the TXA2 receptor β carboxyl-terminal fusion protein. One of three similar experiments is shown in each case.
Figure 3.
In vivo phosphorylation of immunopurified TXA2 receptor by cyclic GMP and the thromboxane analog U46619. TXA2 receptors were isolated by immunoaffinity methods from 32P-labeled HEL cells and resolved on SDS/PAGE gels, transferred to nitrocellulose, and then subjected to autoradiography and immunoblotting. C, vehicle control, 10 min; G, 10 mM 8-Br-cGMP, 15 min; U, 5μM U46619, 10 min. The lower panel is an immunoblot of the nitrocellulose shown phosphorylated in the upper panel with anti-TXA2 receptor antibody. One of five similar experiments is shown.
The third intracytoplasmic loop and the cytoplasmic tails of both the TXA2 receptors α and β all contain potential serine/threonine phosphorylation sites for G kinase, with 2, 6, and 17 serine/threonine residues, respectively (23, 24). Peptide mapping studies of in vivo phosphorylated TXA2 receptors were undertaken next with endoproteinase Lys-C, which cleaves and releases the seventh transmembrane domain and cytoplasmic tail of TXA2 receptors from the main body of the receptor by proteolysis of Lys-288 (42) (Fig. 4). Digestion of in vivo phosphorylated TXA2 receptors with endoproteinase Lys-C resulted in the release of receptor-associated 32P into a small 6–8-kDa carboxyl terminal fragment of the receptor (Fig. 4). Identical results were obtained by Lys-C digestion of immunopurified TXA2 receptors phosphorylated in vitro (data not shown). Little or no phosphorylation was seen associated with any larger Mr proteins following endoproteinase Lys-C digestion in these studies (Fig. 4). These data exclude the intracytoplasmic loops of the TXA2 receptor as the site(s) of phosphorylation by G kinase and map the site(s) to somewhere distal to Lysine 288 in the carboxyl terminus.
Figure 4.
Endoproteinase Lys-C digestion of in vivo phosphorylated TXA2 receptors. Undigested in vivo phosphorylated TXA2 receptors (−) or receptors enzymatically digested by endoproteinase Lys-C (+) and resolved on 15% SDS/PAGE gel. Note that receptor-associated 32P is released by Lys-C digestion and migrates at 6–8 kDa, associated with the carboxyl-terminal tail fragment (“Tail”). F, gel front. One of two similar experiments is shown.
Phosphorylation of the TXA2 Receptor Cytoplasmic Terminal Peptide by G Kinase.
To examine further the potential phosphorylation of cytoplasmic domains of TXA2 receptors, an in vitro phosphorylation assay was used to test the ability of G kinase to catalyze the phosphorylation of GST-fusion proteins derived from the third intracytoplasmic loop and C-terminal domains of the TXA2 receptors (Fig. 5). Native GST protein (G, left and right panels) was not phosphorylated in these studies, nor was the peptide derived from the third intracytoplasmic loop of the TXA2 receptor phosphorylated (iL3, left panel), even when concentrations of these two fusion proteins 5–10-fold higher than those of the carboxyl-terminal peptide-derived fusion protein were used (data not shown). In contrast, physiologic concentrations of G kinase (8 nM) markedly catalyzed the phosphorylation of the fusion peptide corresponding to the cytoplasmic tail of the TXA2 receptor α (Fig. 5, left and right panels). Phosphorylation of the α receptor carboxyl-terminal protein was dose-dependent and maximal at a G kinase concentration of approximately 40 nM (data not shown). In separate studies, peptide corresponding to the cytoplasmic tail of the TXA2 receptor β also was phosphorylated using G kinase (Fig. 5, right panel, Lane B). The level of phosphorylation of the α and β isoforms of the TXA2 receptor carboxyl termini was quite similar in this assay, although the β form has 15 more serine/threonine residues than the α form available for phosphorylation.
DISCUSSION
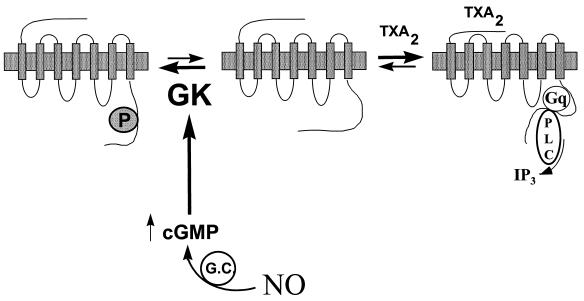
The data presented here show that cGMP directly inhibits TXA2-specific GTPase activity in platelet membranes and that G kinase catalyzes phosphorylation of the cytoplasmic carboxyl-terminal domain of the thromboxane receptor. These data support a new model for inhibition of the TXA2 receptor–G protein–PLC–InsP3 signaling pathway by NO and cGMP (Fig. 6). This model is consistent with previous reports demonstrating NO- and cGMP-mediated inhibition of agonist-induced InsP3 generation and [Ca2+]i mobilization in platelets and vascular smooth muscle cells (16, 52, 53) and has direct parallels to the agonist-activated desensitization of G protein-coupled receptors by the family of GRK (see refs. 34 and 36). However, in contrast to homologous desensitization by GRK, our data demonstrate receptor phosphorylation by G kinase without a requirement for prior agonist occupancy (although it remains possible that G kinase phosphorylates a precoupled receptor population in our studies). In the model proposed in Fig. 6, inhibition of G protein receptor signaling is triggered by NO-mediated increases in intracellular cGMP levels in resting cells, which results in basal receptor phosphorylation. NO thus would shift the equilibrium in resting cells between uncoupled receptors (R) and those receptors functionally coupled to their cognate G protein(s) (R*) prior to agonist exposure (Fig. 6; cf. refs. 54–58) and could render the receptor less able to be activated by agonist (R−).
Figure 6.
Proposed model for the inhibition of G protein-coupled receptor activation by nitric oxide and cyclic-GMP. Nitric oxide activates guanylate cyclase, producing cGMP and activating G kinase (GK). G kinase in turn phosphorylates the TXA2 receptor, which prevents or disrupts coupling of the receptor to its cognate GTP-binding protein Gq and thus inhibits activation of the effector, phospholipase C (PLC), preventing [Ca2+]i mobilization and cellular activation. The net effect is to shift the equilibrium in resting cells between precoupled receptor and uncoupled receptor toward the uncoupled state. It is also possible that the agonist-activated receptor is a target for G kinase. In this model, NO “sets the gain” for cellular activation by G protein-coupled receptors like the TXA2 receptor, resulting in a net decrease in platelet or vascular smooth muscle activation by physiologic agonists.
Cyclic GMP is capable of activating the cAMP-dependent protein kinase (59), and we cannot exclude the possibility that some of the inhibitory effects of 8-Br-cGMP on the platelet TXA2 GTPase in these studies were mediated by cAMP-dependent protein kinase. However, previous data support that these inhibitory effects of cGMP, as well as those of cAMP, are mediated by G kinase and not cAMP-dependent protein kinase (9, 40, 43). Although our in vitro and in vivo data are consistent in demonstrating incorporation of 32P into the carboxyl-terminal tail of the TXA2 receptors, identification of the specific TXA2 receptor C-terminal serine/threonine residue(s) phosphorylated by G kinase will require sequential site-directed mutagenesis of these serine/threonine residues in future studies. The experiments reported here do not exclude the possibility that for other G protein-coupled receptors inhibited by NO, the G protein and/or its effector are direct targets for phosphorylation by G kinase. For instance, it has been suggested that Gαi is a substrate for G kinase (60). In these latter studies, however, Gi was a relatively poor substrate for G kinase in vitro and in the intact cell, and in our studies we have not been able to demonstrate any increase in phosphorylation of either platelet G proteins or PLC isoforms by NO or cGMP. Our data do not explore whether other proteins involved in G protein-coupled receptor signaling such as the recently appreciated RGS proteins (61) are G kinase substrates, nor do they address whether G kinase phosphorylation recruits additional proteins, like β-arrestin or a homologous protein, to the phosphorylated TXA2 receptors.
The in vitro phosphorylation studies presented raise the possibility that G kinase catalyzes the phosphorylation of amino acid residue(s) in both the α and β receptor isoforms. The TXR2 antibody used in the present work cannot distinguish between these two TXA2 receptor isoforms, and it will therefore also be important to study the degree to which each isoform is a G kinase substrate and which TXRα and TXRβ residues are phosphorylated by G kinase. Based on the data presented here and on previously described consensus phosphorylation sequences for G kinase (62), serine 324 and/or threonine 325, in the amino acid sequence 317-RRLQPRLST-325, are potential candidates for the residue(s) phosphorylated by G kinase in the carboxyl terminus of the TXA2 α receptor.
In vivo, NO is basally secreted and is the most important known endogenous vasodilator, with a central role in the maintenance of normal blood pressure and the inhibition of platelet activation (1, 63). The present data and model predict that normal NO secretion by the endothelium maintains some degree of G kinase activation above baseline and thus a basal level of phosphorylation of smooth muscle cell and platelet TXA2 receptors, which might be lost in disease states characterized by decreased NO elaboration such as hypercholesterolemia and atherosclerosis (1). In addition, stimulated increases in NO and cGMP would be predicted to increase the level of receptor phosphorylation. Thus, in vivo, both basal and stimulated NO secretion would lead to rightward shifts of the dose-response curves for receptor-coupled cellular responses, “setting the gain” of the TXA2 platelet activation pathway with potentially important physiological consequences. Future studies will test this model further for the thromboxane receptor and for other members of the G protein-coupled receptor family.
Acknowledgments
We are grateful to Drs. Shuh Narumiya and Masakazu Hirata for providing us with the cDNA for the human TXA2 receptor α and to Dr. Tony Ware for the TXA2 β receptor cDNA. We thank Drs. Maria A. Diversé-Pierluissi, Kathleen Dunlap, and Richard H. Karas for critical review of the manuscript, Wendy Baur for expert handling of the cell cultures used in this study, and Patricia G. Nayak for expert preparation of the manuscript. This work was supported in part by National Institutes of Health Grant HL36838 (P.V.H.), a South Carolina affiliate American Heart Association grant (P.V.H.), National Institutes of Health Grant HL55309 (M.E.M.), and a grant-in-aid from the National Center of the American Heart Association (M.E.M.). M.E.M. is an Established Investigator of the American Heart Association.
ABBREVIATIONS
- G kinase
cGMP-dependent protein kinase
- TXA2
thromboxane A2
- InsP3
inositol trisphosphate
- PLC
phospholipase C
- GRK
G protein receptor kinases
- GST
glutathione S-transferase
- [Ca2+]i
intracellular calcium
- 8-Br-cGMP
8-bromo-cyclic GMP
References
- 1.Bredt D S, Snyder S H. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 2.Schafer A I, Alexander R W, Handin R I. Blood. 1980;55:649–654. [PubMed] [Google Scholar]
- 3.Mellion B T, Ignarro L J, Ohlstein E H, Pontecorvo E G, Hyman A L, Kadowitz P J. Blood. 1981;57:946–955. [PubMed] [Google Scholar]
- 4.Azuma H, Ishikawa M, Sekizaki S. Br J Pharmacol. 1986;88:411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mollace V, Salvemini D, Anggard E, Vane J. Br J Pharmacol. 1991;104:633–638. doi: 10.1111/j.1476-5381.1991.tb12481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieberman E H, O’Neill S, Mendelsohn M E. Circ Res. 1991;68:1722–1728. doi: 10.1161/01.res.68.6.1722. [DOI] [PubMed] [Google Scholar]
- 7.Sneddon J M, Vane J R. Proc Natl Acad Sci USA. 1988;85:2800–2804. doi: 10.1073/pnas.85.8.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendelsohn M E, O’Neill S, George D, Loscalzo J. J Biol Chem. 1990;265:19028–19034. [PubMed] [Google Scholar]
- 9.Lincoln T M. Cyclic GMP: Biochemistry, Physiology and Pathophysiology. Austin, TX: R. G. Landes; 1994. [Google Scholar]
- 10.Morgan R O, Newby A C. Biochem J. 1989;258:447. doi: 10.1042/bj2580447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takai Y, Kaibuchi K, Matsubara T, Nishizuka Y. Biochem Biophys Res Commun. 1981;101:61–67. doi: 10.1016/s0006-291x(81)80010-1. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima S, Tohmatsu T, Hattori H, Okano Y, Nozawa Y. Biochem Biophys Res Commun. 1986;135:1099–1104. doi: 10.1016/0006-291x(86)91041-7. [DOI] [PubMed] [Google Scholar]
- 13.Waldmann R, Walter U. Eur J Pharmacol. 1989;159:317–320. doi: 10.1016/0014-2999(89)90165-9. [DOI] [PubMed] [Google Scholar]
- 14.Durante W, Kroll M H, Vanhoutte P M, Schafer A I. Blood. 1992;79:110–116. [PubMed] [Google Scholar]
- 15.Geiger J, Nolte C, Butt E, Sage S O, Walter U. Proc Natl Acad Sci USA. 1992;89:1031–1035. doi: 10.1073/pnas.89.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruth P, Wang G-X, Boekhoff I, May B, Pfeifer A, Penner R, Korth M, Breer H, Hofmann F. Proc Natl Acad Sci USA. 1993;90:2623–2627. doi: 10.1073/pnas.90.7.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moro M A, Russell R J, Cellek S, Lizasoain I, Su Y, Darley-Usmar V, Radomski M, Moncada S. Proc Natl Acad Sci USA. 1996;93:1480–1485. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oates J A, FitzGerald G A, Branch R A, Jackson E K, Knapp H R, Roberts L J. N Engl J Med. 1988;319:761–767. doi: 10.1056/NEJM198809223191206. [DOI] [PubMed] [Google Scholar]
- 19.Arita H, Nakano T, Hanasaki K. Prog Lipid Res. 1989;28:273–301. doi: 10.1016/0163-7827(89)90002-7. [DOI] [PubMed] [Google Scholar]
- 20.Ware J A, Heistad D D. N Engl J Med. 1993;328:628–635. doi: 10.1056/NEJM199303043280907. [DOI] [PubMed] [Google Scholar]
- 21.Cairns J A. Cardiovasc Clin. 1987;18:231–246. [PubMed] [Google Scholar]
- 22.Hirata M, Hayaski Y, Ushikubi F, Nakanishi S, Narumiya S. Nature (London) 1991;349:617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- 23.Raychowdhury M K, Yukawa M, Collins L J, McGrail S H, Kent K C, Ware J A. J Biol Chem. 1994;269:19256–19261. , and correction (1995) 270, 7011. [PubMed] [Google Scholar]
- 24.Hirata T, Ushikubi F, Kakizuka A, Okuma M, Narumiya S. J Clin Invest. 1996;97:949–956. doi: 10.1172/JCI118518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brass L F, Shaller C C, Belmonte E J. J Clin Invest. 1987;79:1269–1275. doi: 10.1172/JCI112947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knezevic I, Borg C, Le Breton G C. J Biol Chem. 1993;268:26011–26017. [PubMed] [Google Scholar]
- 27.Shenker A, Goldsmith P, Unson C G, Spiegel A M. J Biol Chem. 1991;266:9309–9313. [PubMed] [Google Scholar]
- 28.Offermanns S, Laugwitz K-L, Spicher K, Schultz G. Proc Natl Acad Sci USA. 1994;91:504–508. doi: 10.1073/pnas.91.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benka M L, Lee M, Wang G R, Busa W B, Mendelsohn M E The MBL Physiology Course. FEBS Lett. 1995;363:49–52. [Google Scholar]
- 30.Reilly, M. & FitzGerald, G. A. (1993) Cellular activation of thromboxane A2 and other eicosanoids. Eur. Heart J. 14, K88–K93. [PubMed]
- 31.Iafrati M D, Karas R H, Aronovitz M, Kim S, Sullivan T R, Jr, Lubhan D B, O’Donnell T F, Jr, Korach K S, Mendelsohn M E. Nat Med. 1997;3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 32.Offermanns S, Toombs C F, Hu Y, Simon M I. Nature (London) 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 33.Kroll M H. In: Thrombosis and Hemorrhage. Loscalzo J, Schafer A I, editors. Vol. 13. Oxford: Blackwell Scientific; 1994. pp. 247–277. [Google Scholar]
- 34.Hausdorff W P, Caron M G, Lefkowitz R J. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 35.Spiegel A M, Shenker A, Weinstein L S. Endocr Rev. 1992;13:536–565. doi: 10.1210/edrv-13-3-536. [DOI] [PubMed] [Google Scholar]
- 36.Lefkowitz R J. Cell. 1993;74:409–412. doi: 10.1016/0092-8674(93)80042-d. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Y, Tassi L, Lane W, Mendelsohn M E. J Biol Chem. 1994;269:22379–22384. [PubMed] [Google Scholar]
- 38.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 1–726. [Google Scholar]
- 39.Mendelsohn M E, Zhu Y, O’Neill S. Proc Natl Acad Sci USA. 1991;88:11212–11216. doi: 10.1073/pnas.88.24.11212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lincoln, T. M., Cornwell, T. L. & Taylor, A. E. (1989) Am. J. Physiol. 399–407.
- 41.Woolkalis M J, Nakada M T, Manning D R. J Biol Chem. 1986;261:3408–3413. [PubMed] [Google Scholar]
- 42.True T A, Mais D E. Eur J Pharmacol. 1994;266:51–55. doi: 10.1016/0922-4106(94)90208-9. [DOI] [PubMed] [Google Scholar]
- 43.Cornwell T L, Lincoln T M. J Biol Chem. 1989;264:1146–1155. [PubMed] [Google Scholar]
- 44.Lincoln T M, Cornwell T L, Komalavilas P, Boerth N. Methods Enzymol. 1996;269:149–166. doi: 10.1016/s0076-6879(96)69017-x. [DOI] [PubMed] [Google Scholar]
- 45.Allan C J, Higashiura K, Martin M, Morinelli T A, Kurtz D T, Geoffroy O, Meier G P, Gettys T W, Halushka P V. J Pharmacol Exp Ther. 1996;277:1132–1139. [PubMed] [Google Scholar]
- 46.Mayeux P R, Mais D E, Carr C, Halushka P V. J Pharmacol Exp Ther. 1989;250:923–927. [PubMed] [Google Scholar]
- 47.Schror K, Davis-Bruno K, Halushka P V. Biochem Pharmacol. 1995;49:921–927. doi: 10.1016/0006-2952(95)00015-r. [DOI] [PubMed] [Google Scholar]
- 48.Harrington, M. G. (1990) ed. Deutscher, M. P., pp. 488–495.
- 49.Albers F J, Middleton J P. Naunyn-Schmiedeberg’s Arch Pharmacol. 1992;346:127–137. doi: 10.1007/BF00165293. [DOI] [PubMed] [Google Scholar]
- 50.Lincoln T M, Corbin J D. Adv Cyclic Nucleotide Res. 1983;15:192. [PubMed] [Google Scholar]
- 51.Mais D E, True T A, Martinelli M J. Eur J Pharmacol. 1992;227:267–274. doi: 10.1016/0922-4106(92)90004-f. [DOI] [PubMed] [Google Scholar]
- 52.Waldmann R, Bauer S, Gobel C, Hofmann F, Jakobs K H, Walter U. Eur J Biochem. 1986;158:203–210. doi: 10.1111/j.1432-1033.1986.tb09739.x. [DOI] [PubMed] [Google Scholar]
- 53.Felbel J, Trockur B, Ecker T, Landgraf W, Hofmann F. J Biol Chem. 1988;263:16764–16771. [PubMed] [Google Scholar]
- 54.Bond R A, Leff P, Johnson T D, Milano C A, Rockman H A, McMinn T R, Apparsundaram S, Hyek M F, Kenakin T P, Allen L F, Lefkowitz R J. Nature (London) 1995;374:272–275. doi: 10.1038/374272a0. [DOI] [PubMed] [Google Scholar]
- 55.Prezeau L, Gomeza J, Ahern S, Mary S, Galvez T, Bockaert J, Pin J P. Mol Pharmacol. 1996;49:422–429. [PubMed] [Google Scholar]
- 56.Parker E M, Swigart P, Nunnally M H, Perkins J P, Ross E M. J Biol Chem. 1995;270:6482–6487. doi: 10.1074/jbc.270.12.6482. [DOI] [PubMed] [Google Scholar]
- 57.Tian W N, Duzic E, Lanier S M, Deth R C. Mol Pharmacol. 1994;45:524–531. [PubMed] [Google Scholar]
- 58.Tian W N, Deth R C. Life Sci. 1993;52:1899–1907. doi: 10.1016/0024-3205(93)90630-l. [DOI] [PubMed] [Google Scholar]
- 59.Cornwell T L, Arnold E, Boerth N J, Lincoln T M. Am J Physiol. 1994;267:C1405–C1413. doi: 10.1152/ajpcell.1994.267.5.C1405. [DOI] [PubMed] [Google Scholar]
- 60.Pfeifer A, Nurnberg B, Kamm S, Uhde M, Schultz G, Ruth P, Hofmann F. J Biol Chem. 1995;270:9052–9059. doi: 10.1074/jbc.270.16.9052. [DOI] [PubMed] [Google Scholar]
- 61.Berman D M, Wilkie T M, Gilman A G. Cell. 1996;86:445–452. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 62.Pearson R B, Kemp B E. In: Protein Kinase Phosphorylation Site Sequences and Consensus Specificity Motifs: Tabulations. Hunter T, Sefton B M, editors. San Diego: Academic; 1991. pp. 62–84. [DOI] [PubMed] [Google Scholar]
- 63.Moncada S, Palmer R M J, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]