Abstract
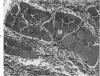
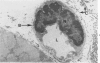
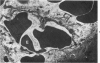
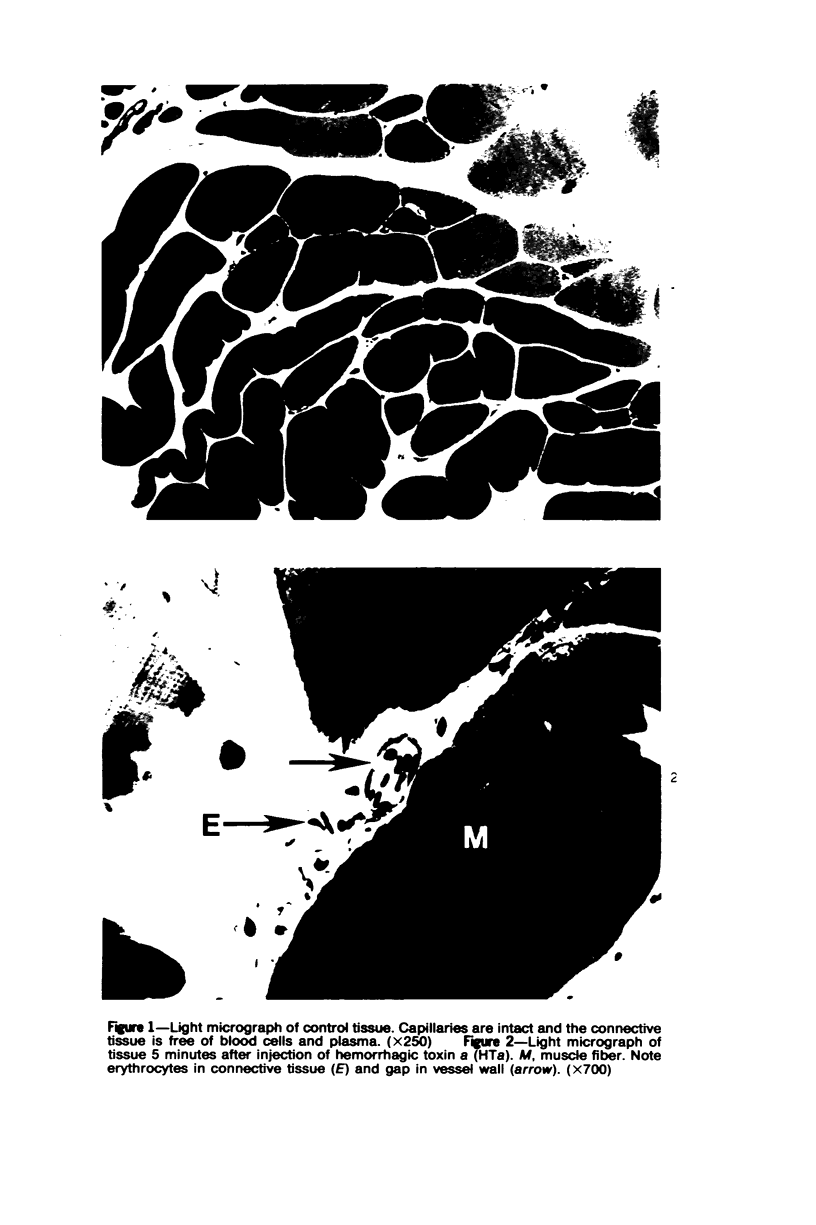
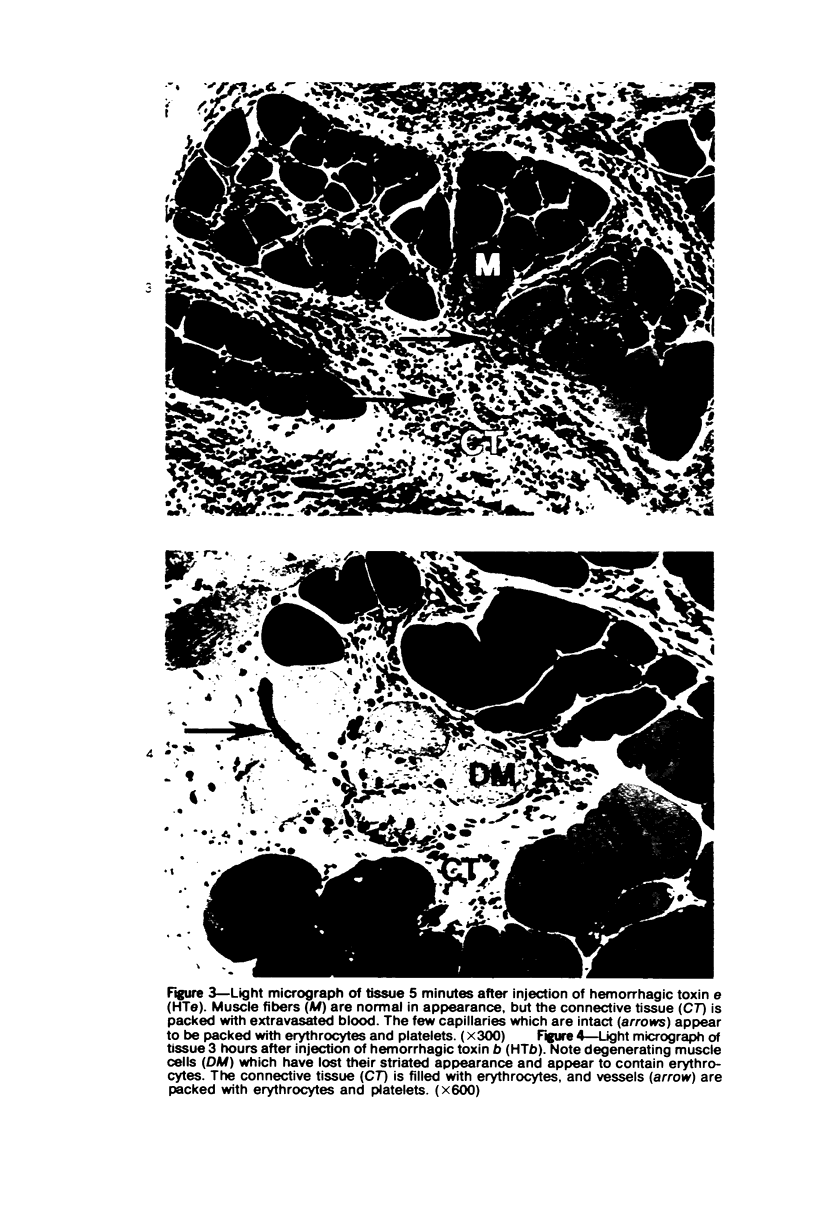
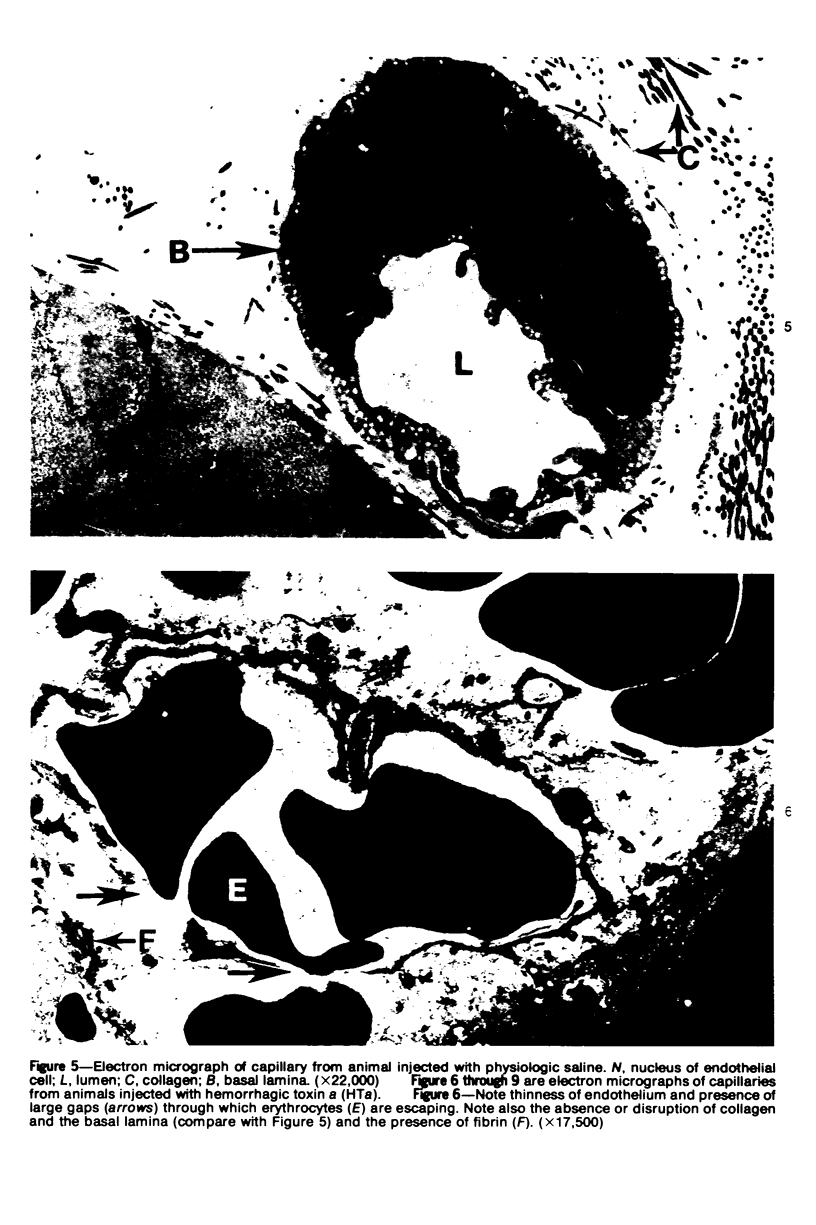
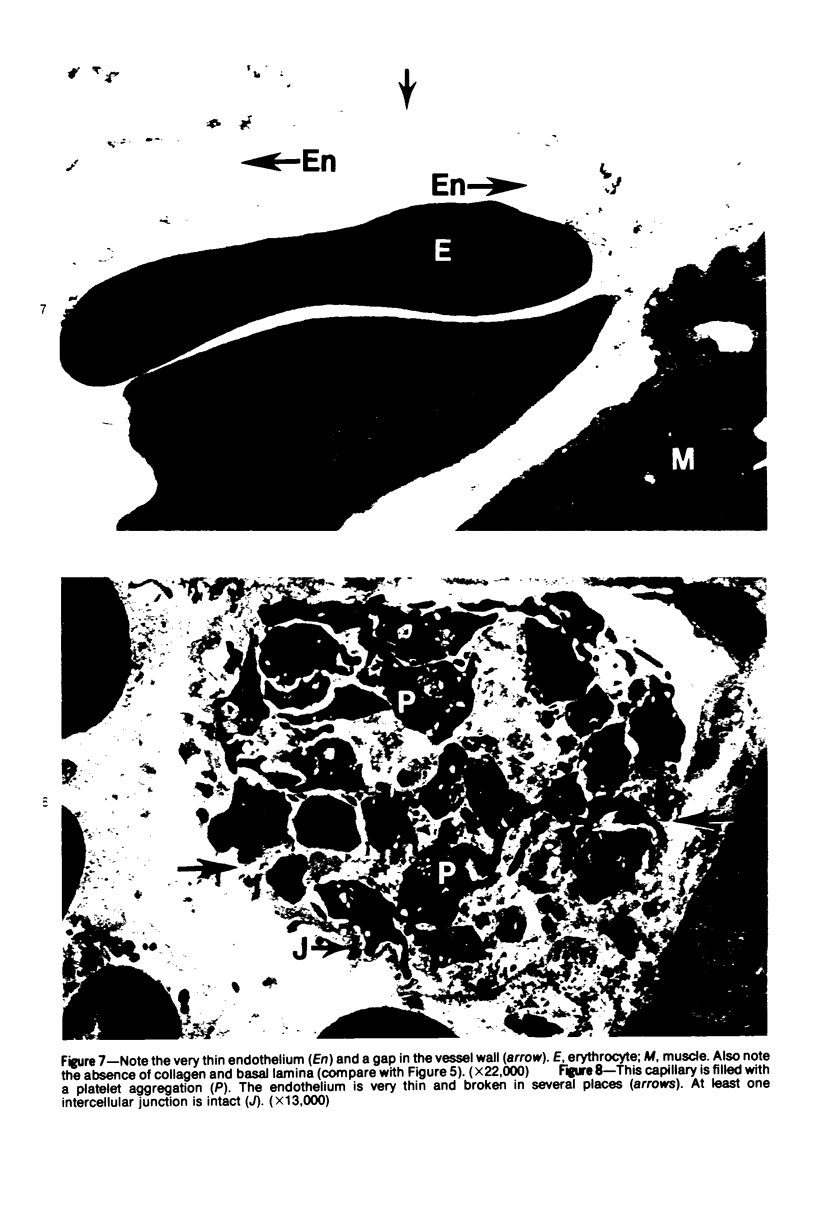
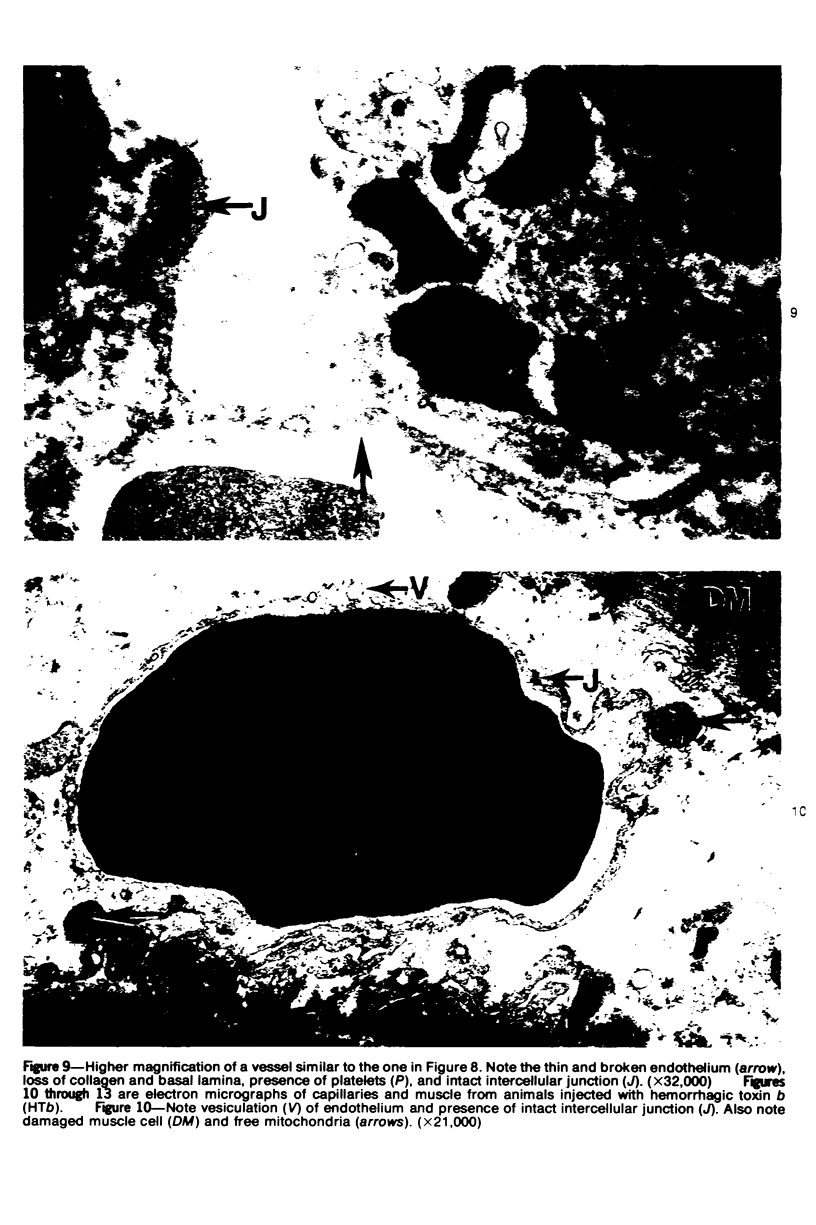
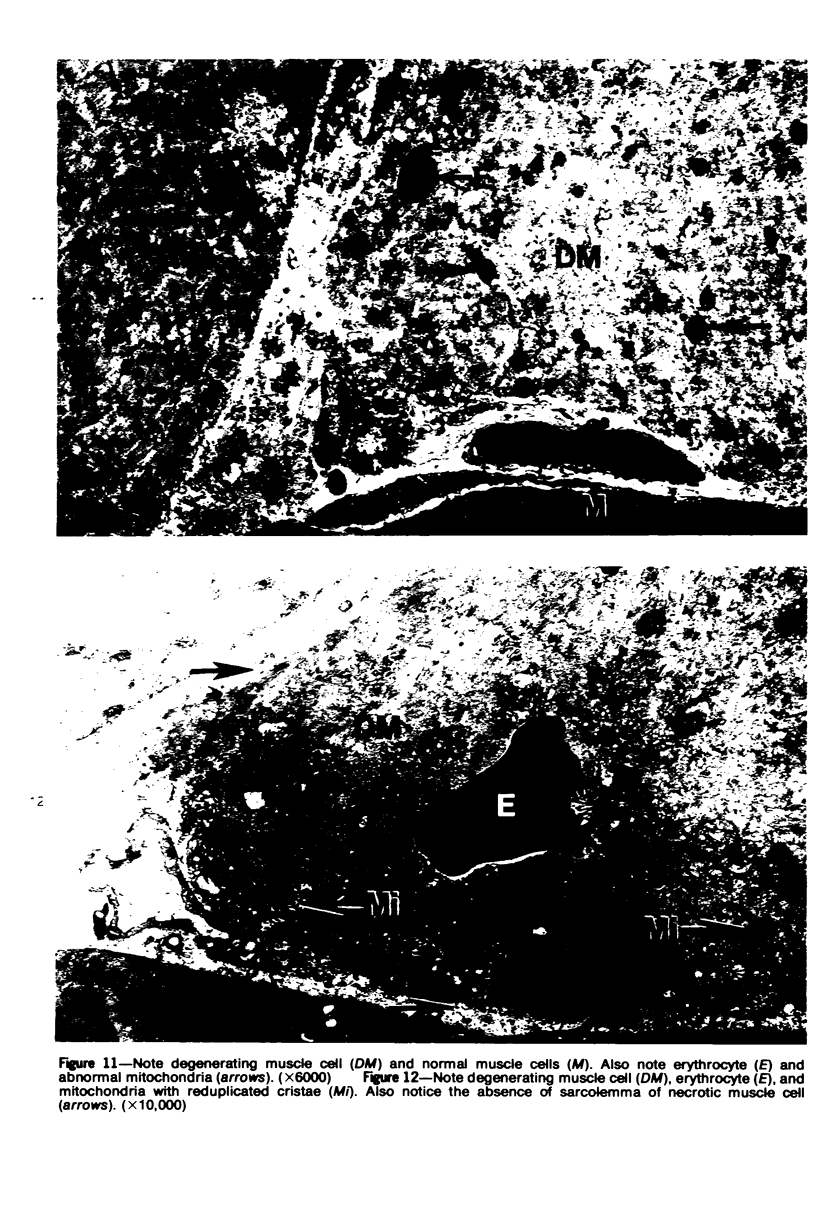
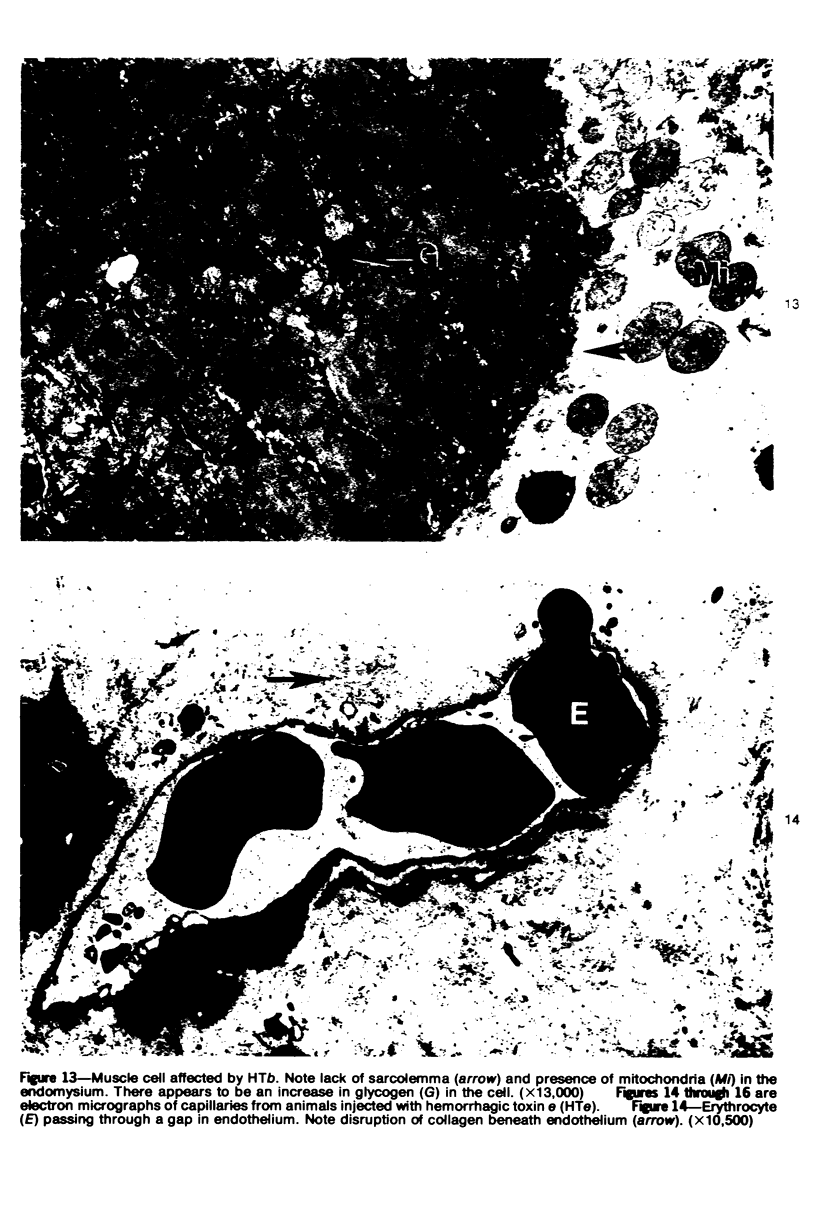
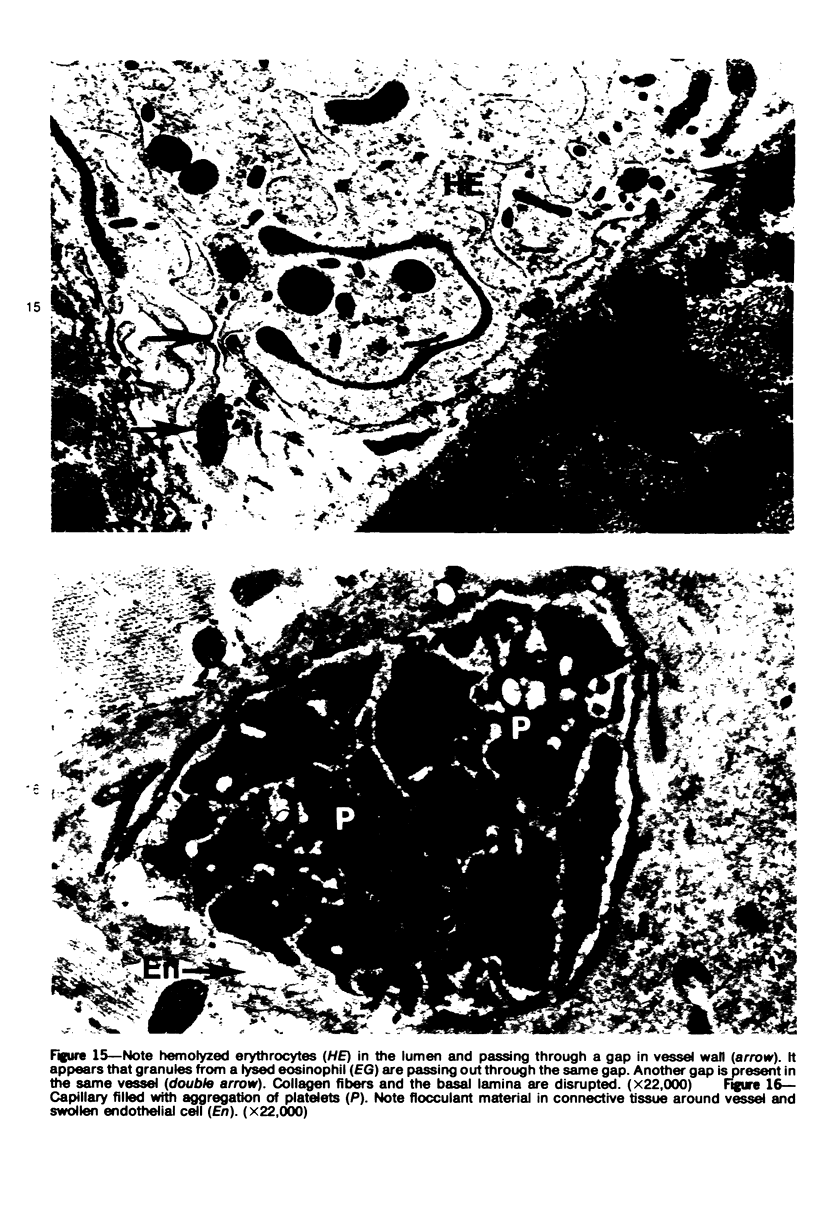
The pathogenesis of hemorrhage induced by three purified components of rattlesnake (Crotalus atrox) venom was studied at the light and electron microscopic levels. Crude venom was fractionated by anion exchange and gel filtration in four steps. beta-Alanine acetate disk gel electrophoresis was used to demonstrate electrophoretic homogeneity. White mice were injected intramuscularly with 0.1 ml of a sublethal dose of hemorrhagic toxin. Gross examination revealed extensive hemorrhage 5 minutes after the injection of hemorrhagic toxins alpha and episilon; the same amount of hemorrhage was not present until 3 hours after the injection of hemorrhagic toxin beta. Light microscopic examination of muscel after injection of the toxins revealed areas of extensive hemorrhage in which very few intact capillaries could be found and also adjacent areas of slight hemorrhage in which capillaries were in various stages of degeneration. Necrosis of muscle cells was evident in tissue injected with hemorrhagic toxin beta. Electron microscopic examination showed that capillaries from toxin-injected muscle were in various stages of degeneration. Endothelial cells became very thin and broke down into vesicles prior to complete rupture. Gaps were formed within the cells while intercellular junctions remained intact. Plasma and erythrocytes leaked through these gaps and were observed in the endomysium. Many gaps were plugged with platelet aggregations. Collagen and the basal lamina associated with capillaries were usually disorganized or absent. The experimental injection of three purified hemorrhagic toxins induced hemorrhage by the same mechanism as does the crude venom, ie, per rhexis. In addition, one of the toxins, hemorrhagic toxin beta, causes myonecrosis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron D. L., Tu A. T. Characterization of myotoxin a from the venom of prairie rattlesnake (Crotalus viridis viridis). Biochemistry. 1977 May 31;16(11):2546–2553. doi: 10.1021/bi00630a035. [DOI] [PubMed] [Google Scholar]
- Grotto L., Moroz C., De Vries A., Goldblum N. Isolation of Vipera palestinae hemorrhagin and distinction between its hemorrhagic and proteolytic activities. Biochim Biophys Acta. 1967 Feb 21;133(2):356–362. doi: 10.1016/0005-2795(67)90075-x. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- KONDO H., KONDO S., IKEZAWA H., MURATA R. Studies on the quantitative method for determination of hemorrhagic activity of Habu snake venom. Jpn J Med Sci Biol. 1960;13:43–52. doi: 10.7883/yoken1952.13.43. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYNN J. A. RAPID TOLUIDINE BLUE STAINING OF EPON-EMBEDDED AND MOUNTED "ADJACENT" SECTIONS. Am J Clin Pathol. 1965 Jul;44:57–58. doi: 10.1093/ajcp/44.1.57. [DOI] [PubMed] [Google Scholar]
- McKay D. G., Moroz C., De Vries A., Csavossy I., Cruse V. The action of hemorrhagin and phospholipase derived from Vipera palestinae venom on the microcirculation. Lab Invest. 1970 May;22(5):387–399. [PubMed] [Google Scholar]
- McKay D. G., Moroz C., De Vries A., Csavossy I., Cruse V. The action of hemorrhagin and phospholipase derived from Vipera palestinae venom on the microcirculation. Lab Invest. 1970 May;22(5):387–399. [PubMed] [Google Scholar]
- Nikai T., Sugihara H., Tanaka T. [Enzymochemical studies on snake venoms. II. Purification of lethal protein Ac1-proteinase in the venom of Agkistrodon acutus (author's transl)]. Yakugaku Zasshi. 1977 May;97(5):507–514. doi: 10.1248/yakushi1947.97.5_507. [DOI] [PubMed] [Google Scholar]
- OMORI T., IWANAGA S., SUZUKI T. THE RELATIONSHIP BETWEEN THE HEMORRHAGIC AND LETHAL ACTIVITIES OF JAPANESE MAMUSHI (AGKISTRODON HALYS BLOMHOFFII) VENOM. Toxicon. 1964 Jun;15:1–4. doi: 10.1016/0041-0101(64)90027-3. [DOI] [PubMed] [Google Scholar]
- Omori-Satoh T., Ohsaka A. Purification and some properties of hemorrhagic principle I in the venom of Trimeresurus flavoviridis. Biochim Biophys Acta. 1970 Jun 23;207(3):432–444. doi: 10.1016/s0005-2795(70)80006-x. [DOI] [PubMed] [Google Scholar]
- Osaka A., Just M., Habermann E. Action of snake venom hemorrhagic principles on isolated glomerular basement membrane. Biochim Biophys Acta. 1973 Oct 25;323(3):415–428. doi: 10.1016/0005-2736(73)90187-9. [DOI] [PubMed] [Google Scholar]
- Osaka A., Oashi M., Tsuchiya M., Kamisaka Y., Fujishiro Y. Action of Trimeresurus flavoviridis venom on the microcirculatory system of rat; dynamic aspects as revealed by cinephotomicrographic recording. Jpn J Med Sci Biol. 1971 Feb;24(1):34–39. [PubMed] [Google Scholar]
- Osaka A., Suzuki K., Oashi M. The spurting of erythrocytes through junctions of the vascular endothelium treated with snake venom. Microvasc Res. 1975 Sep;10(2):208–213. doi: 10.1016/0026-2862(75)90007-2. [DOI] [PubMed] [Google Scholar]
- Oshima G., Iwanaga S., Suzuki T. Studies on snake venoms. 18. An improved method for purification of the proteinase b from the venom of Agkistrodon halys blomhoffii and its physicochemical properties. J Biochem. 1968 Aug;64(2):215–225. doi: 10.1093/oxfordjournals.jbchem.a128882. [DOI] [PubMed] [Google Scholar]
- Oshima G., Omori-Sato T., Iwanaga S., Suzuki T. Studies on snake venom hemorrhagic factor I (HR-I) in the venom of Agkistrodon halys blomhoffi. Its purification and biological properties. J Biochem. 1972 Dec;72(6):1483–1494. doi: 10.1093/oxfordjournals.jbchem.a130040. [DOI] [PubMed] [Google Scholar]
- Ownby C. L., Cameron D., Tu A. T. Isolation of myotoxic component from rattlesnake (Crotalus viridis viridis) venom. Electron microscopic analysis of muscle damage. Am J Pathol. 1976 Oct;85(1):149–166. [PMC free article] [PubMed] [Google Scholar]
- Ownby C. L., Kainer R. A., Tu A. T. Pathogenesis of hemorrhage induced by rattlesnake venom. An electron microscopic study. Am J Pathol. 1974 Aug;76(2):401–414. [PMC free article] [PubMed] [Google Scholar]
- Sandbank U., Jerushalmy Z., Ben-David E., De Vries A. Effect of Echis coloratus venom on brain vessels. Toxicon. 1974 May;12(3):267–271. doi: 10.1016/0041-0101(74)90068-3. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stringer J. M., Kainer R. A., Tu A. T. Myonecrosis induced by rattlesnake venom. An electron microscopic study. Am J Pathol. 1972 Apr;67(1):127–140. [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Osaka A. Purification and some properties of two hemorrhagic principles (HR2a and HR2b) in the venom of Trimeresurus flavoviridis; complete separation of the principles from proteolytic activity. Biochim Biophys Acta. 1970 Apr 28;207(1):65–75. doi: 10.1016/0005-2795(70)90137-6. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]