Abstract
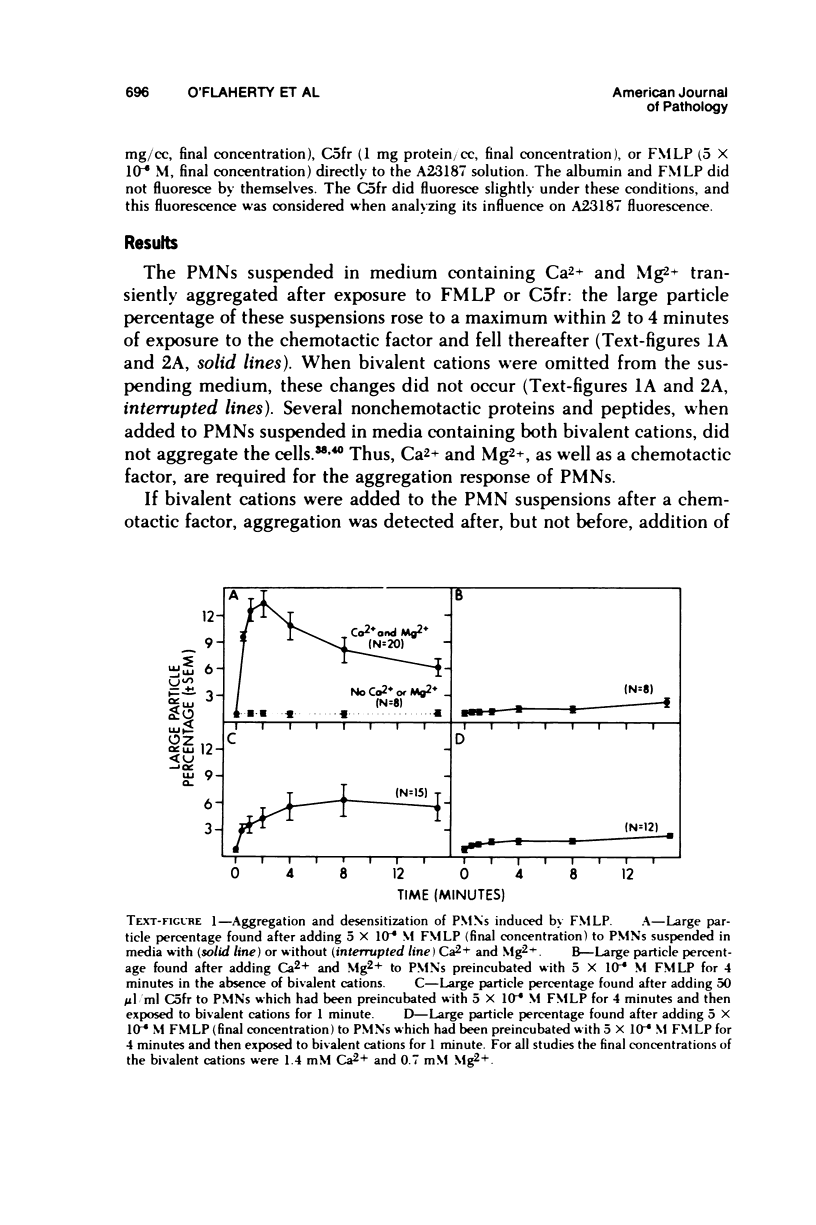
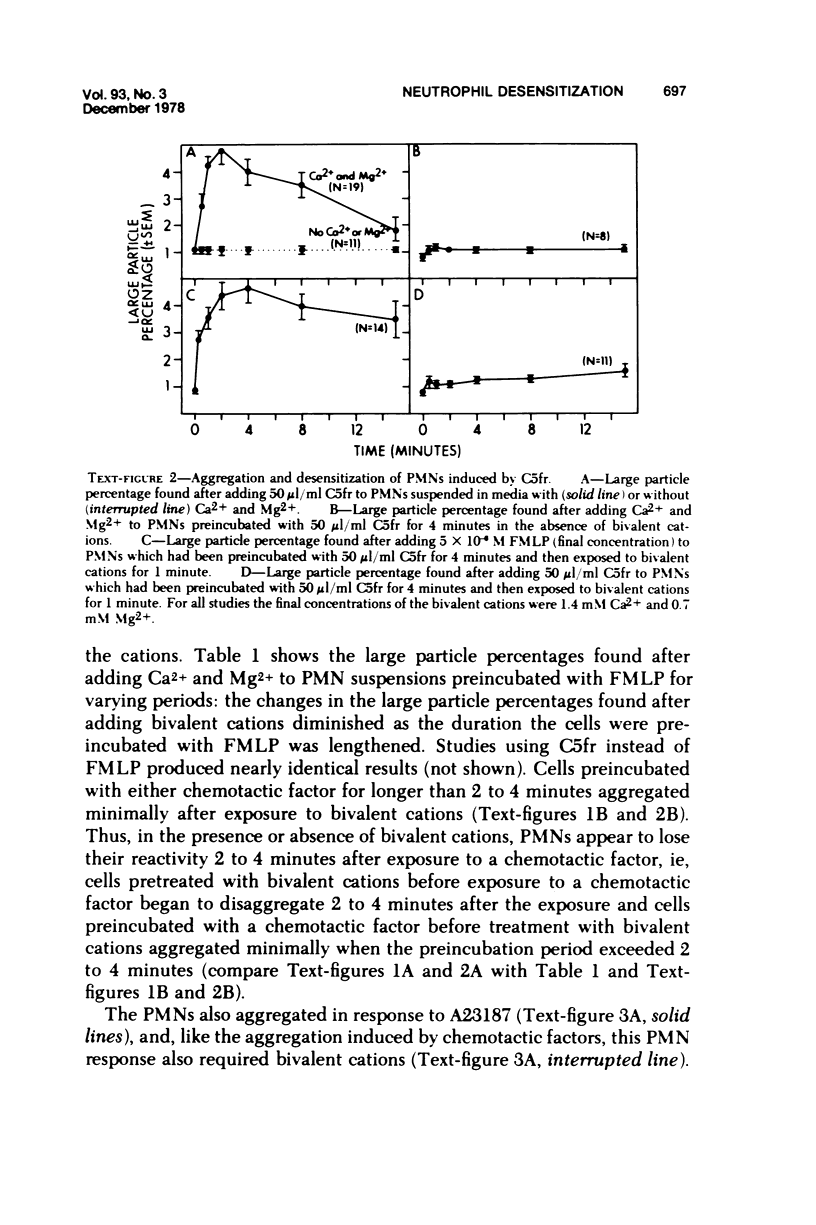
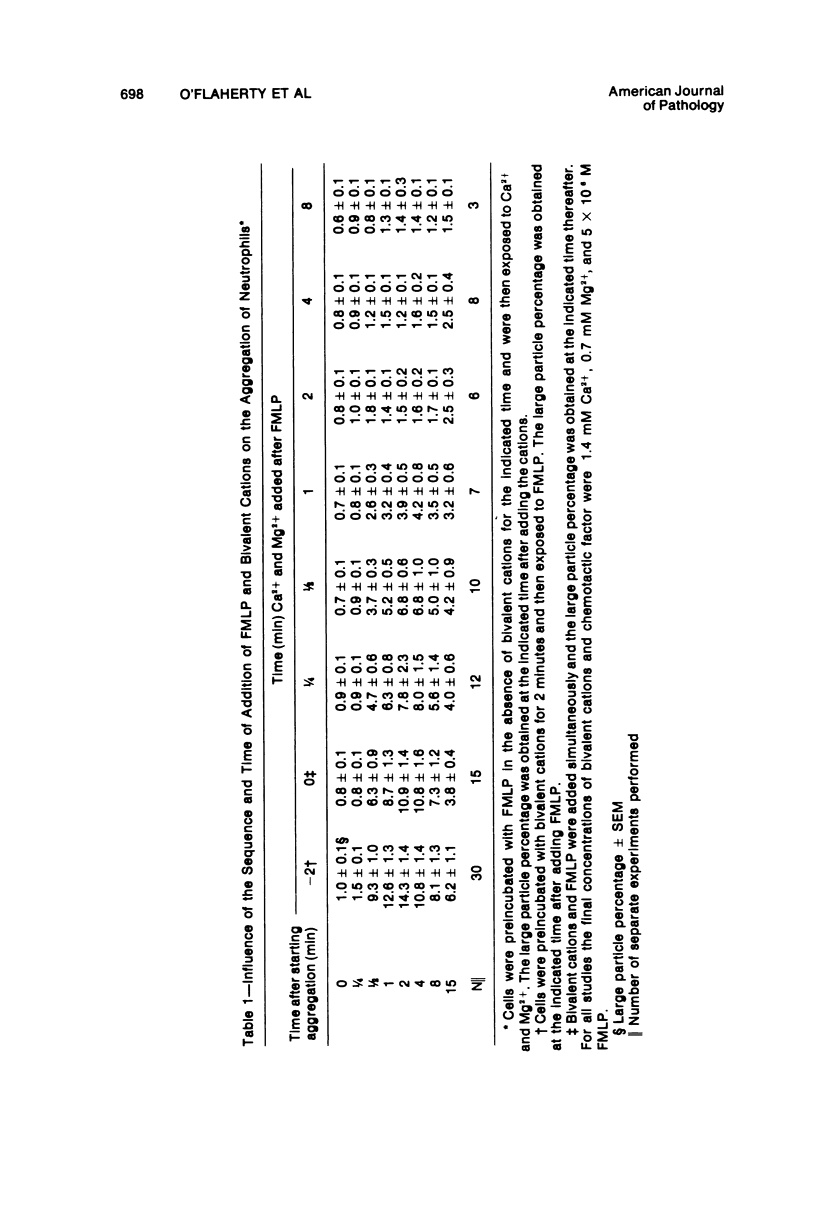
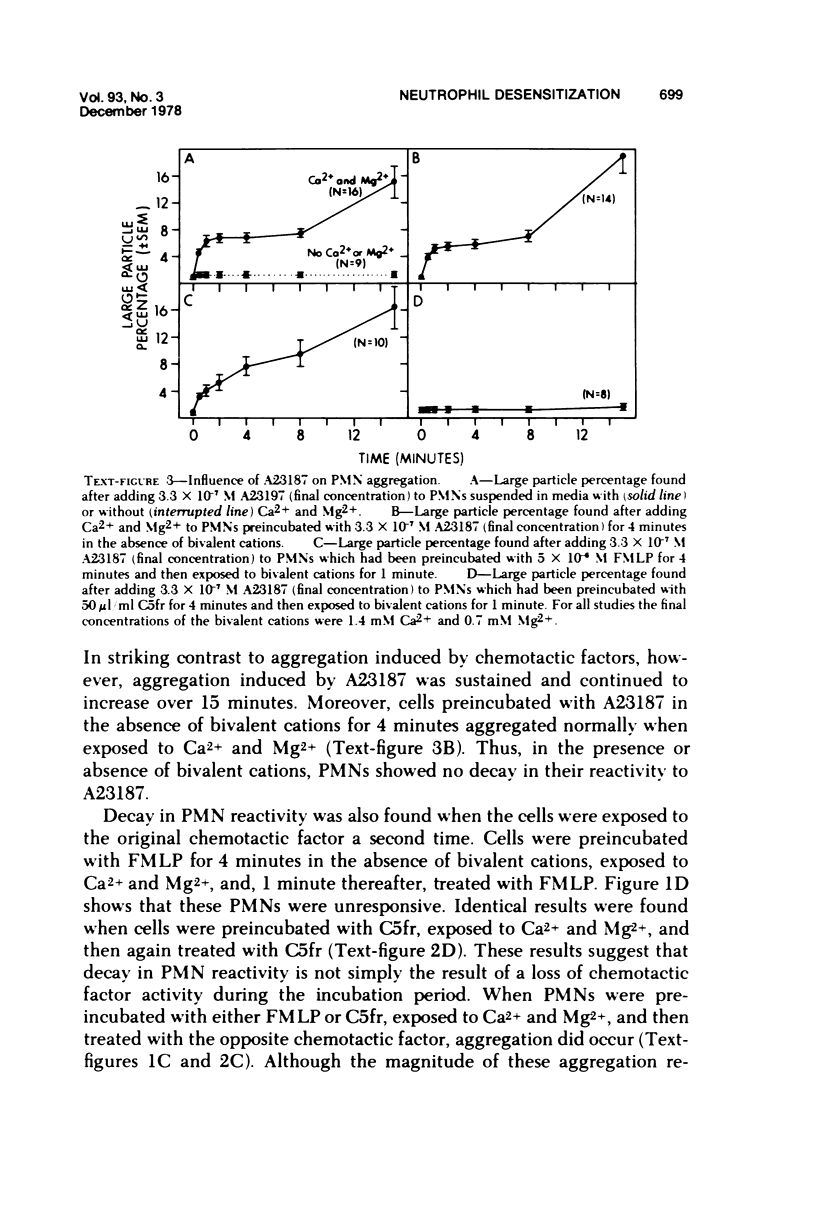
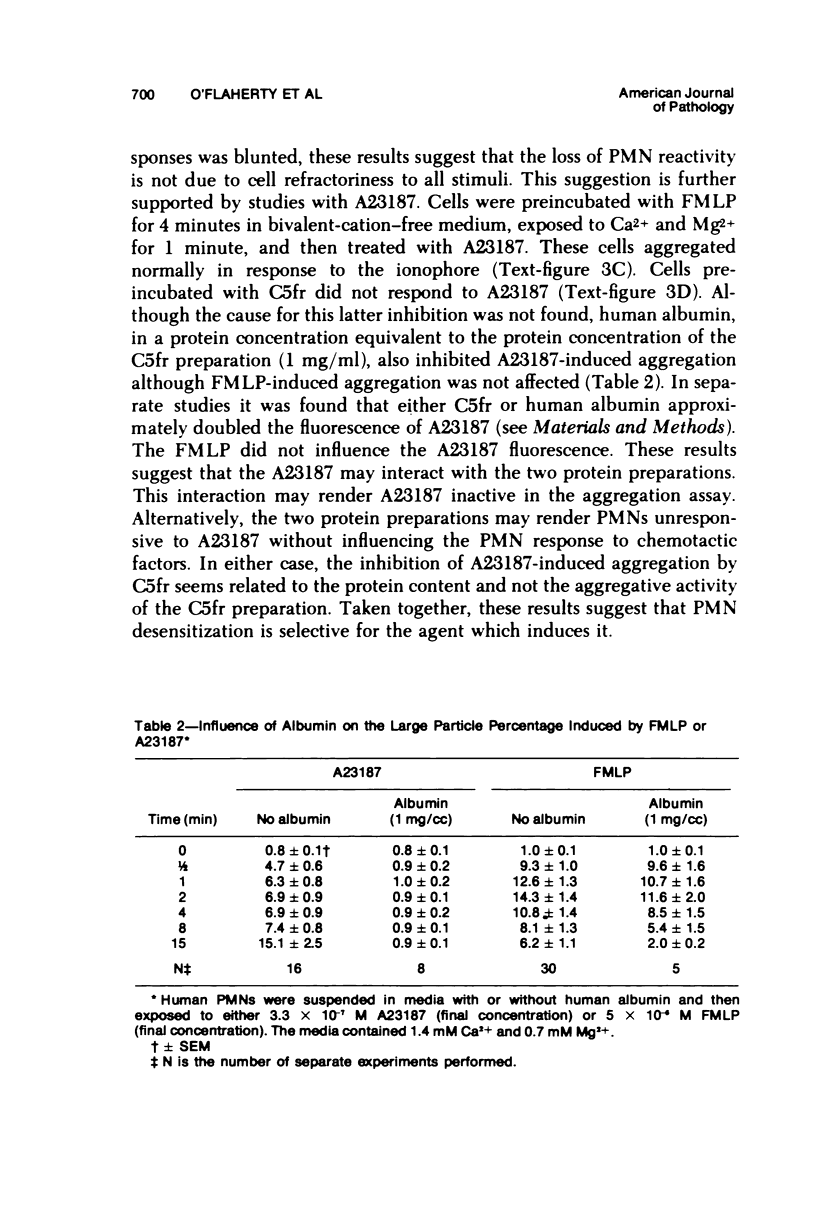
In the presence of Ca2+ and Mg2+, the chemotactic fragment of C5, the synthetic chemotactic oligopeptide formyl-methionyl-leucyl-phenyl-alanine, and the ionophore A23187 aggregated human neutrophils. Aggregation induced by the two chemotactic factors was transient and reversed within 2 to 4 minutes after exposure; aggregation induced by A23187 was sustained and continued to increase over 15 minutes. In the absence of the bivalent cations, none of these three agents aggregated the cells. If bivalent cations were added after cell contact with a chemotactic factor, aggregation was detected after, but not before, addition of the cations. Under these conditions, the magnitude of the aggregation response was sharply reduced: cells preincubated with a chemotactic factor for longer than 2 to 4 minutes aggregated minimally after addition of bivalent cations. Moreover, cells preincubated with a chemotactic factor for 4 minutes, exposed to bivalent cations, and then rechallenged with the same chemotactic factor also showed a minimal aggregation response, ie, the cells were "desensitized" to the original stimulus. However, cells desensitized to one of the chemotactic factors still aggregated prominently when exposed to the other chemotactic factor or to A23187. Cells could not be desensitized to the ionophore A23187. Desensitization of the neutrophil aggregation response closely resembles desensitization of mast cell and leukocyte degranulation. Degranulation and aggregation appear to be closely related cellular responses to immunologic stimuli. Both responses may reflect alterations in surface membrane permeability to bivalent cations and/or changes in surface membrane adhesiveness to other biologic membranes.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswanikumar S., Schiffmann E., Corcoran B. A., Wahl S. M. Role of a peptidase in phagocyte chemotaxis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2439–2442. doi: 10.1073/pnas.73.7.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. H., Adamik R. Control of histamine release: effects of various conditions on rate of release and rate of cell desensitization. J Immunol. 1975 Mar;114(3):1034–1041. [PubMed] [Google Scholar]
- Becker E. L., Showell H. J. The ability of chemotactic factors to induce lysosomal enzyme release. II. The mechanism of release. J Immunol. 1974 Jun;112(6):2055–2062. [PubMed] [Google Scholar]
- Boucek M. M., Snyderman R. Calcium influx requirement for human neutrophil chemotaxis: inhibition by lanthanum chloride. Science. 1976 Sep 3;193(4256):905–907. doi: 10.1126/science.948752. [DOI] [PubMed] [Google Scholar]
- Case G. D., Vanderkooi J. M., Scarpa A. Physical properties of biological membranes determined by the fluorescence of the calcium ionophore A23187. Arch Biochem Biophys. 1974 May;162(1):174–185. doi: 10.1016/0003-9861(74)90116-7. [DOI] [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48-80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci U S A. 1974 Feb;71(2):408–412. doi: 10.1073/pnas.71.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist R. Relationship of uptake of sodium and 45calcium to ATP-induced histamine release from rat mast cells. Acta Pharmacol Toxicol (Copenh) 1974 Jul;35(1):11–22. doi: 10.1111/j.1600-0773.1974.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Diamant B., Grosman N., Stahl Skov P., Thomle S. Effect of divalent cations and metabolic energy on the anaphylactic histamine release from rat peritoneal mast cells. Int Arch Allergy Appl Immunol. 1974;47(3):412–424. doi: 10.1159/000231234. [DOI] [PubMed] [Google Scholar]
- Estensen R. D., Reusch M. E., Epstein M. L., Hill H. R. Role of Ca2+ and Mg2+ in some human neutrophil functions as indicated by ionophore A23187. Infect Immun. 1976 Jan;13(1):146–151. doi: 10.1128/iai.13.1.146-151.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr J., Jacob H. S. In vitro granulocyte adherence and in vivo margination: two associated complement-dependent functions. Studies based on the acute neutropenia of filtration leukophoresis. J Exp Med. 1977 Sep 1;146(3):641–652. doi: 10.1084/jem.146.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Garland L. G. Desensitization in the process of histamine secretion induced by antigen and dextran. J Physiol. 1974 Jun;239(2):381–391. doi: 10.1113/jphysiol.1974.sp010574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. Proceedings: 45-Calcium uptake in rat peritoneal mast cells. Br J Pharmacol. 1975 Oct;55(2):283P–284P. [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The interaction of calcium and strontium with phosphatidyl serine in the anaphylactic secretion of histamine. J Physiol. 1973 Apr;230(2):493–507. doi: 10.1113/jphysiol.1973.sp010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland L. G., Mongar J. L. Differential histamine release by dextran and the ionophore A23187: the actions of inhibitors. Int Arch Allergy Appl Immunol. 1976;50(1):27–42. doi: 10.1159/000231477. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Hoffstein S. T., Weissmann G. Influence of divalent cations upon complement-mediated enzyme release from human polymorphonuclear leukocytes. J Immunol. 1975 Sep;115(3):665–670. [PubMed] [Google Scholar]
- Goldstein I. M., Horn J. K., Kaplan H. B., Weissmann G. Calcium-induced lysozyme secretion from human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1974 Sep 23;60(2):807–812. doi: 10.1016/0006-291x(74)90312-x. [DOI] [PubMed] [Google Scholar]
- Grant J. A., Dupree E., Goldman A. S., Schultz D. R., Jackson A. L. Complement-mediated release of histamine from human leukocytes. J Immunol. 1975 Mar;114(3):1101–1106. [PubMed] [Google Scholar]
- LICHTENSTEIN L. M., OSLER A. G. STUDIES ON THE MECHANISMS OF HYPERSENSITIVITY PHENOMENA. IX. HISTAMINE RELEASE FROM HUMAN LEUKOCYTES BY RAGWEED POLLEN ANTIGEN. J Exp Med. 1964 Oct 1;120:507–530. doi: 10.1084/jem.120.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein L. M. The immediate allergic response: in vitro separation of antigen activation, decay and histamine release. J Immunol. 1971 Oct;107(4):1122–1130. [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Changes in ionic movements across rabbit polymorphonuclear leukocyte membranes during lysosomal enzyme release. Possible ionic basis for lysosomal enzyme release. J Cell Biol. 1977 Dec;75(3):635–649. doi: 10.1083/jcb.75.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Showell H. J., Ward P. A. Influence of inhibitors of cellular function on chemotactic factor-induced neutrophil aggregation. J Immunol. 1977 Nov;119(5):1751–1756. [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Chemotactic factor influences on the aggregation, swelling, and foreign surface adhesiveness of human leukocytes. Am J Pathol. 1978 Mar;90(3):537–550. [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Neutrophil aggregation and swelling induced by chemotactic agents. J Immunol. 1977 Jul;119(1):232–239. [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. The influence of chemotactic factors on neutrophil adhesiveness. Inflammation. 1978 Mar;3(1):37–48. doi: 10.1007/BF00917320. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Becker E. L., Ward P. A. Substances which aggregate neutrophils. Mechanism of action. Am J Pathol. 1978 Jul;92(1):155–166. [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Kreutzer D. L., Ward P. A., Becker E. L. Inhibition of in vivo and in vitro neutrophil responses to chemotactic factors by a competitive antagonist. J Immunol. 1978 Apr;120(4):1326–1332. [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Ward P. A. Influence of extracellular Ca2+ and Mg2+ on chemotactic factor-induced neutrophil aggregation. Inflammation. 1977 Dec;2(4):265–276. doi: 10.1007/BF00921006. [DOI] [PubMed] [Google Scholar]
- Petersson B. A., Nilsson A., Stålenheim G. Induction of histamine release and desensitization in human leukocytes. Effect of anaphylatoxin. J Immunol. 1975 May;114(5):1581–1584. [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Showell H. J., Naccache P. H., Sha'afi R. I., Becker E. L. The effects of extracellular K+, Na+ and Ca++ on lysosomal enzyme secretion from polymorphonuclear leukocytes. J Immunol. 1977 Sep;119(3):804–811. [PubMed] [Google Scholar]
- Siraganian R. P., Hook W. A. Complement-induced histamine release from human basophils. II. Mechanism of the histamine release reaction. J Immunol. 1976 Mar;116(3):639–646. [PubMed] [Google Scholar]
- Siraganian R. P., Hook W. A. Mechanism of histamine release by formyl methionine-containing peptides. J Immunol. 1977 Dec;119(6):2078–2083. [PubMed] [Google Scholar]
- Siraganian R. P., Osler A. G. Antigenic release of histamine from rabbit leukocytes. J Immunol. 1970 Jun;104(6):1340–1347. [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabucchi G., Soranzo M. R., Rossi F. Exocytosis in human polymorphonuclear leukocytes induced by A 23187 and calcium. FEBS Lett. 1975 Jun 1;54(1):44–48. doi: 10.1016/0014-5793(75)81064-7. [DOI] [PubMed] [Google Scholar]



