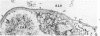Abstract
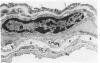
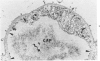
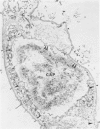
A new method for electron microscopic identification of endothelial basal lamina has been developed by treating fresh human lung tissues with 5 M guanidine HCl, pH 7.2, at 20 C for 24 hours. The guanidine treatment causes significant differential swelling and substantial decrease in electron density of the endothelial basal lamina and makes the latter readily distinguishable from the epithelial basal lamina. In the thin part of alveolar septums, the unit basal lamina shared by the epithelium on one side and the endothelium on the other is found to be composed of discrete epithelial and endothelial layers in close apposition. There is no structural modification at the site of apposition. The epithelial and endothelial basal laminas in alveolar septums seem to be two independent scaffold systems with distinct structural identities despite frequent close physical contact. The findings provide a structural basis for considering separation and apposition of epithelial and endothelial basal laminas in lung reaction to injury and in the pathogenesis of alveolocapillary block.
Full text
PDF







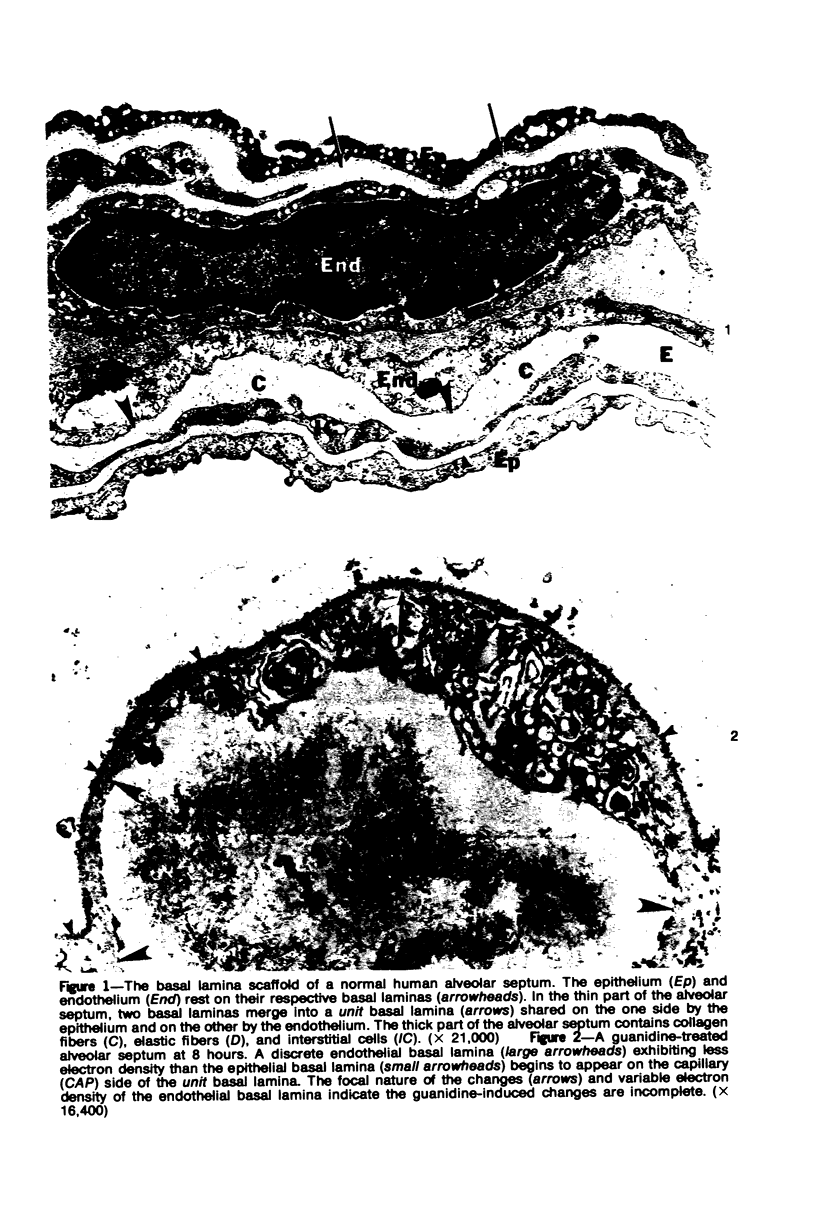
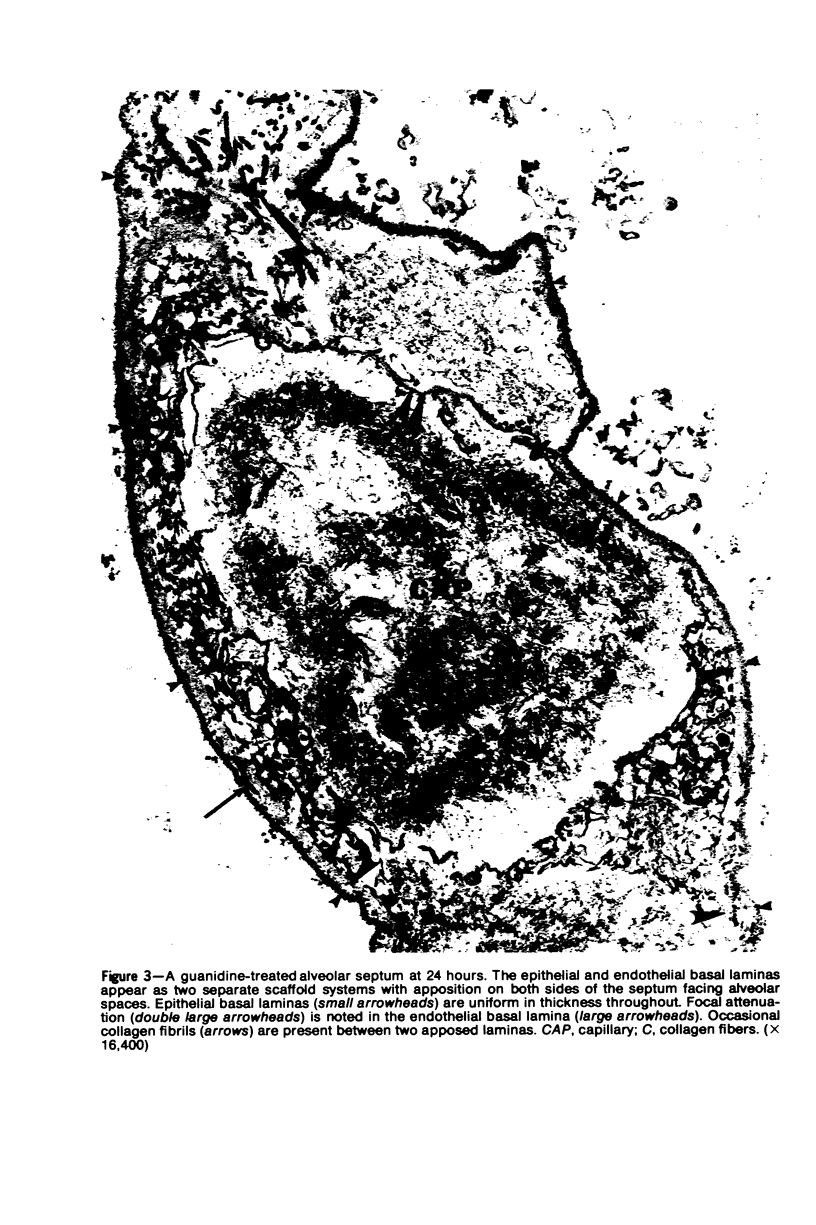
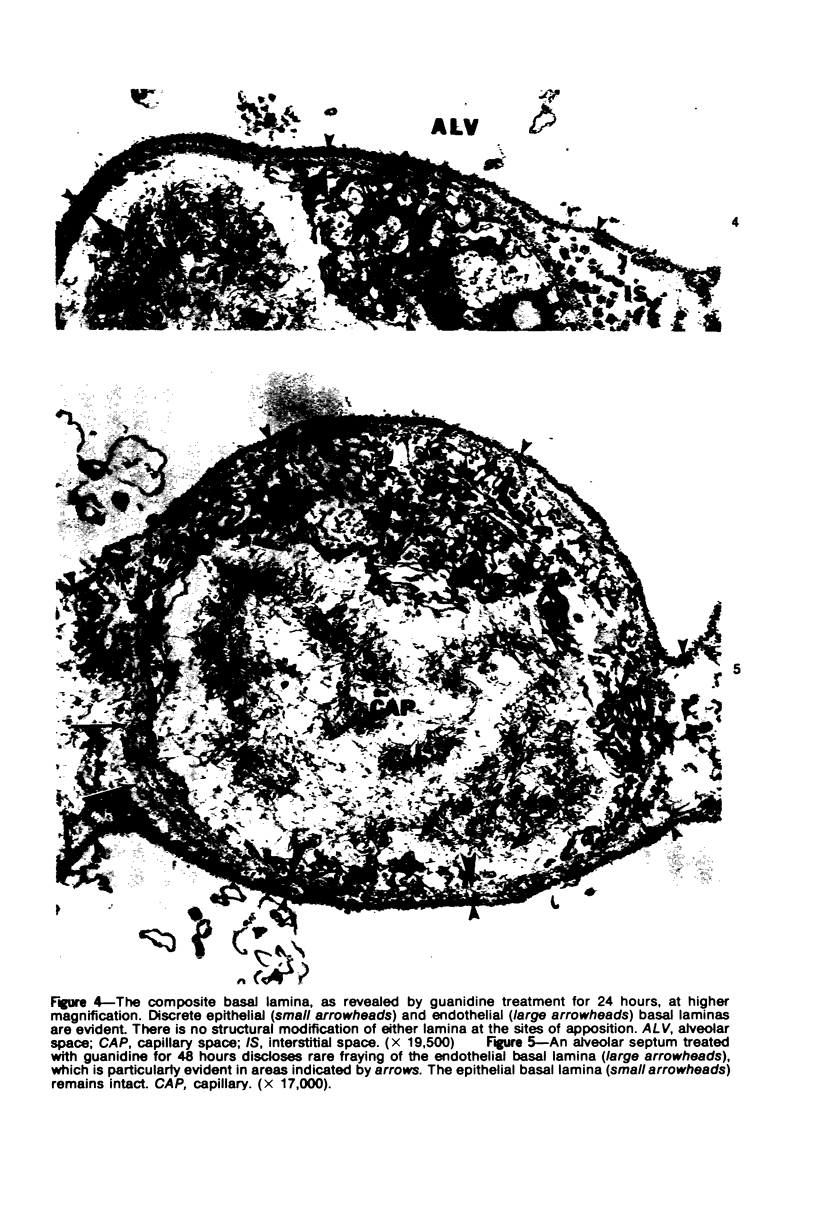
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowden D. H., Wyatt J. P. Lung injury and repair: a contemporary view. Pathol Annu. 1970;5:279–307. [PubMed] [Google Scholar]
- Böhm G. M. The basement membrane in increased permeability [proceedings]. Agents Actions. 1978 Jan;8(1-2):156–157. doi: 10.1007/BF01972424. [DOI] [PubMed] [Google Scholar]
- CRUICKSHANK B., HILL A. G. The histochemical identification of a connective-tissue antigen in the rat. J Pathol Bacteriol. 1953 Jul;66(1):283–289. doi: 10.1002/path.1700660132. [DOI] [PubMed] [Google Scholar]
- Chung E., Rhodes K., Miller E. J. Isolation of three collagenous components of probable basement membrane origin from several tissues. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1167–1174. doi: 10.1016/0006-291x(76)90776-2. [DOI] [PubMed] [Google Scholar]
- Huang T. W., Carlson J. R., Bray T. M., Bradley B. J. 3-methylindole-induced pulmonary injury in goats. Am J Pathol. 1977 Jun;87(3):647–666. [PMC free article] [PubMed] [Google Scholar]
- Huang T. W., Lagunoff D., Benditt E. P. Nonaggregative adherence of platelets to basal lamina in vitro. Lab Invest. 1974 Aug;31(2):156–160. [PubMed] [Google Scholar]
- Huang W. Chemical and histochemical studies of human alveolar collagen fibers. Am J Pathol. 1977 Jan;86(1):81–98. [PMC free article] [PubMed] [Google Scholar]
- KARRER H. E. The ultrastructure of mouse lung; general architecture of capillary and alveolar walls. J Biophys Biochem Cytol. 1956 May 25;2(3):241–252. doi: 10.1083/jcb.2.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res. 1973;6:63–104. doi: 10.1016/b978-0-12-363706-2.50008-8. [DOI] [PubMed] [Google Scholar]
- Lamy M., Fallat R. J., Koeniger E., Dietrich H. P., Ratliff J. L., Eberhart R. C., Tucker H. J., Hill J. D. Pathologic features and mechanisms of hypoxemia in adult respiratory distress syndrome. Am Rev Respir Dis. 1976 Aug;114(2):267–284. doi: 10.1164/arrd.1976.114.2.267. [DOI] [PubMed] [Google Scholar]
- MIDGLEY A. R., Jr, PIERCE G. B., Jr IMMUNOHISTOCHEMICAL ANALYSIS OF BASEMENT MEMBRANES OF THE MOUSE. Am J Pathol. 1963 Dec;43:929–943. [PMC free article] [PubMed] [Google Scholar]
- Nash G., Foley F. D., Langlinais P. C. Pulmonary interstitial edema and hyaline membranes in adult burn patients. Electron microscopic observations. Hum Pathol. 1974 Mar;5(2):149–160. doi: 10.1016/s0046-8177(74)80062-6. [DOI] [PubMed] [Google Scholar]
- Okada Y. Electron microscopic study of interstitial pneumonia, with special reference to alveolar epithelial cells. Acta Pathol Jpn. 1972 Nov;22(4):811–821. doi: 10.1111/j.1440-1827.1972.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Osterby R. Quantitative electron microscopy of the glomerular basement membrane. A methodologic study. Lab Invest. 1971 Jul;25(1):15–24. [PubMed] [Google Scholar]
- PIERCE G. B., Jr, BEALS T. F., RAM J. S., MIDGLEY A. R., Jr BASEMENT MEMBRANES. IV. EPITHELIAL ORGIN AND IMMUNOLOGIC CROSS REACTIONS. Am J Pathol. 1964 Dec;45:929–961. [PMC free article] [PubMed] [Google Scholar]
- Ratliff N. B., Wilson J. W., Hackel D. B., Martin A. M., Jr The lung in hemorrhagic shock. II. Observations on alveolar and vascular ultrastructure. Am J Pathol. 1970 Feb;58(2):353–373. [PMC free article] [PubMed] [Google Scholar]
- Ryan S. F. The structure of the interalveolar septum of the mammalian lung. Anat Rec. 1969 Dec;165(4):467–483. doi: 10.1002/ar.1091650403. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Permeability of intestinal capillaries. Pathway followed by dextrans and glycogens. J Cell Biol. 1972 May;53(2):365–392. doi: 10.1083/jcb.53.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulitzeanu D. Antigenic components of rat connective tissue. Br J Exp Pathol. 1965 Oct;46(5):481–488. [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv Protein Chem. 1970;24:1–95. [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- WEIBEL E. R., KNIGHT B. W. A MORPHOMETRIC STUDY ON THE THICKNESS OF THE PULMONARY AIR-BLOOD BARRIER. J Cell Biol. 1964 Jun;21:367–396. doi: 10.1083/jcb.21.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]