Abstract
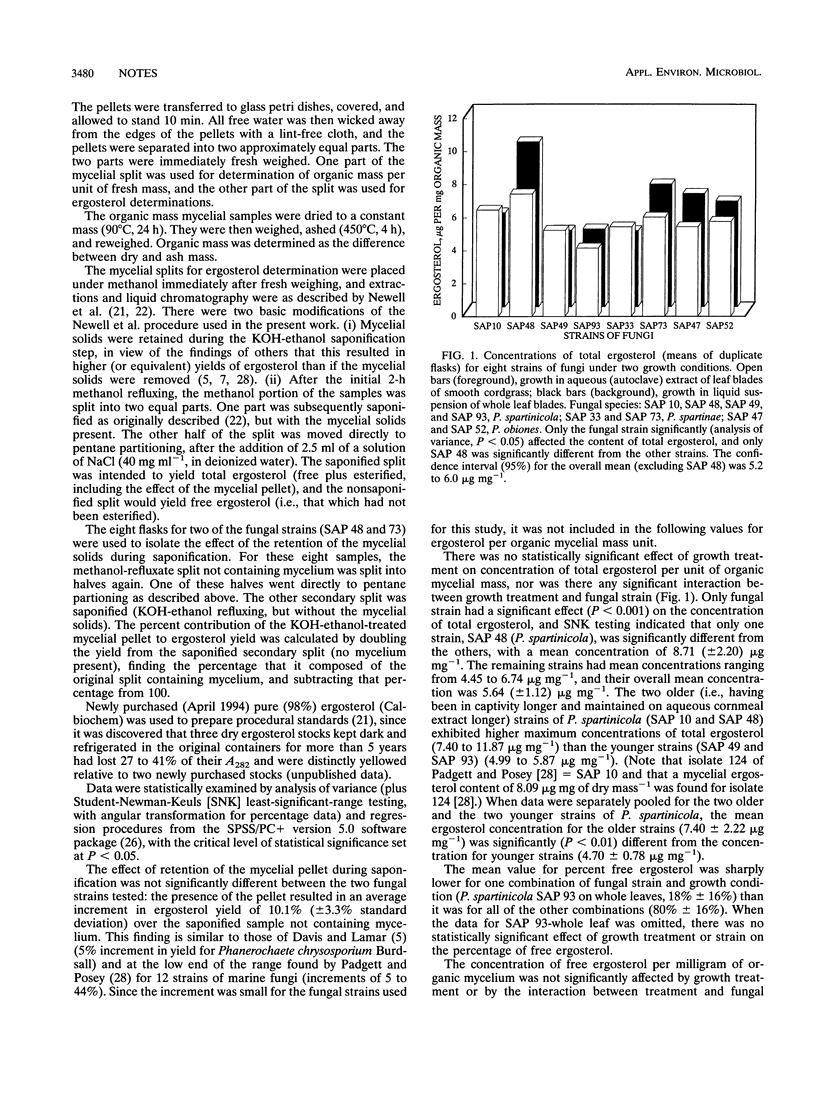
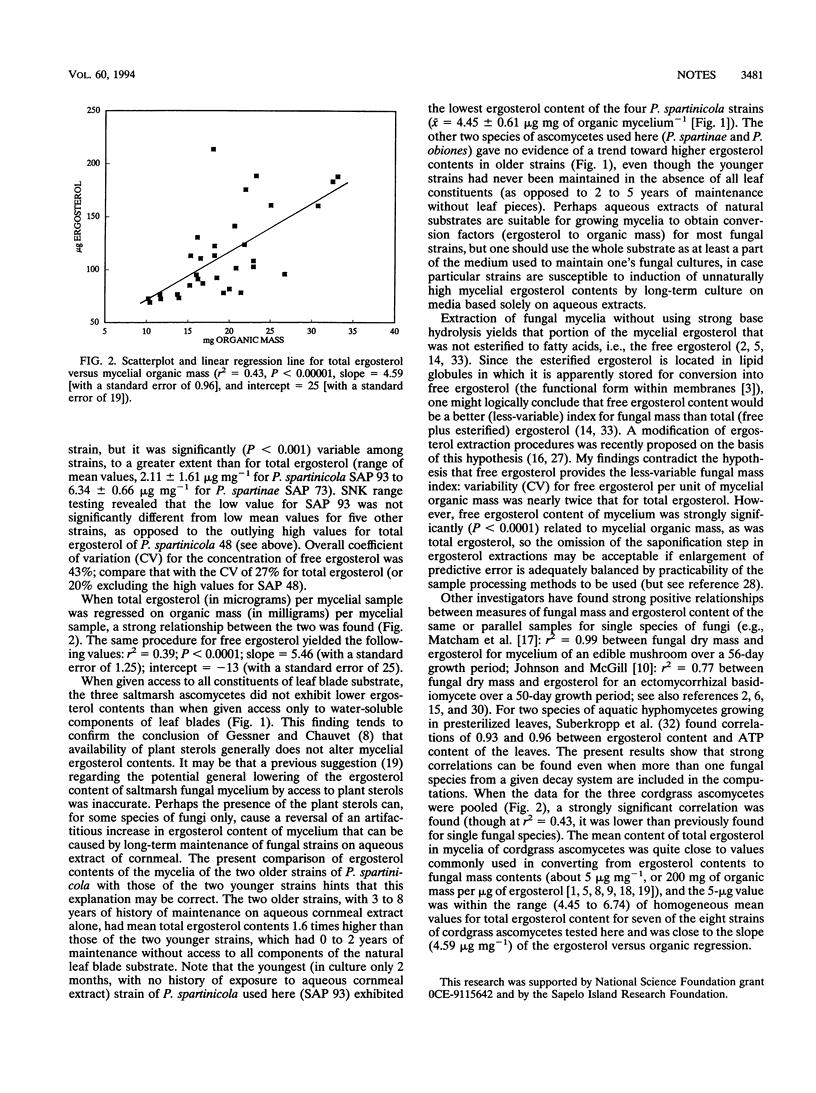
Three species of saltmarsh ascomycetes were grown in the presence of all of the constituents of their natural substrate (leaves of cordgrass) or were presented only with aqueous extracts of the leaves. These two growth-condition treatments had no significant effect on total ergosterol content of the fungal mycelia, contrary to an earlier hypothesis that availability of plant lipids would lower fungal ergosterol contents. Mycelial content of free ergosterol was about twice as variable as that for total (free plus esterified) ergosterol. Total ergosterol (data pooled for all species) was strongly correlated to organic mycelial mass (r2 = 0.43, P < 0.00001, and slope = 4.59 μg of ergosterol mg of organic mass-1).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casey W. M., Keesler G. A., Parks L. W. Regulation of partitioned sterol biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1992 Nov;174(22):7283–7288. doi: 10.1128/jb.174.22.7283-7288.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessner M. O., Chauvet E. Ergosterol-to-Biomass Conversion Factors for Aquatic Hyphomycetes. Appl Environ Microbiol. 1993 Feb;59(2):502–507. doi: 10.1128/aem.59.2.502-507.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R. T., Parks L. W. Involvement of heme components in sterol metabolism of Saccharomyces cerevisiae. Lipids. 1991 Aug;26(8):598–603. doi: 10.1007/BF02536423. [DOI] [PubMed] [Google Scholar]
- Newell S. Y., Arsuffi T. L., Fallon R. D. Fundamental procedures for determining ergosterol content of decaying plant material by liquid chromatography. Appl Environ Microbiol. 1988 Jul;54(7):1876–1879. doi: 10.1128/aem.54.7.1876-1879.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnürer J. Comparison of methods for estimating the biomass of three food-borne fungi with different growth patterns. Appl Environ Microbiol. 1993 Feb;59(2):552–555. doi: 10.1128/aem.59.2.552-555.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suberkropp K., Gessner M. O., Chauvet E. Comparison of ATP and ergosterol as indicators of fungal biomass associated with decomposing leaves in streams. Appl Environ Microbiol. 1993 Oct;59(10):3367–3372. doi: 10.1128/aem.59.10.3367-3372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser E., Paltauf F., Daum G. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J Bacteriol. 1993 May;175(10):2853–2858. doi: 10.1128/jb.175.10.2853-2858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]



