Abstract
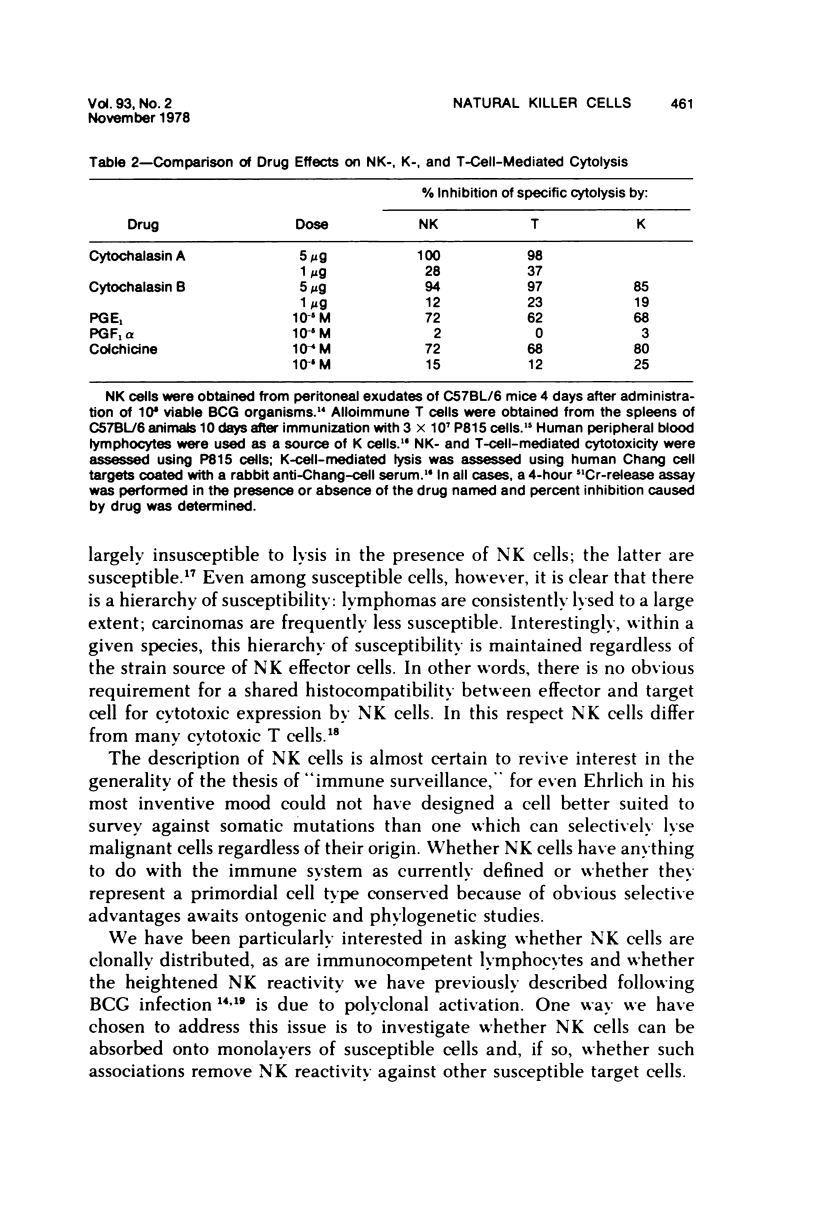
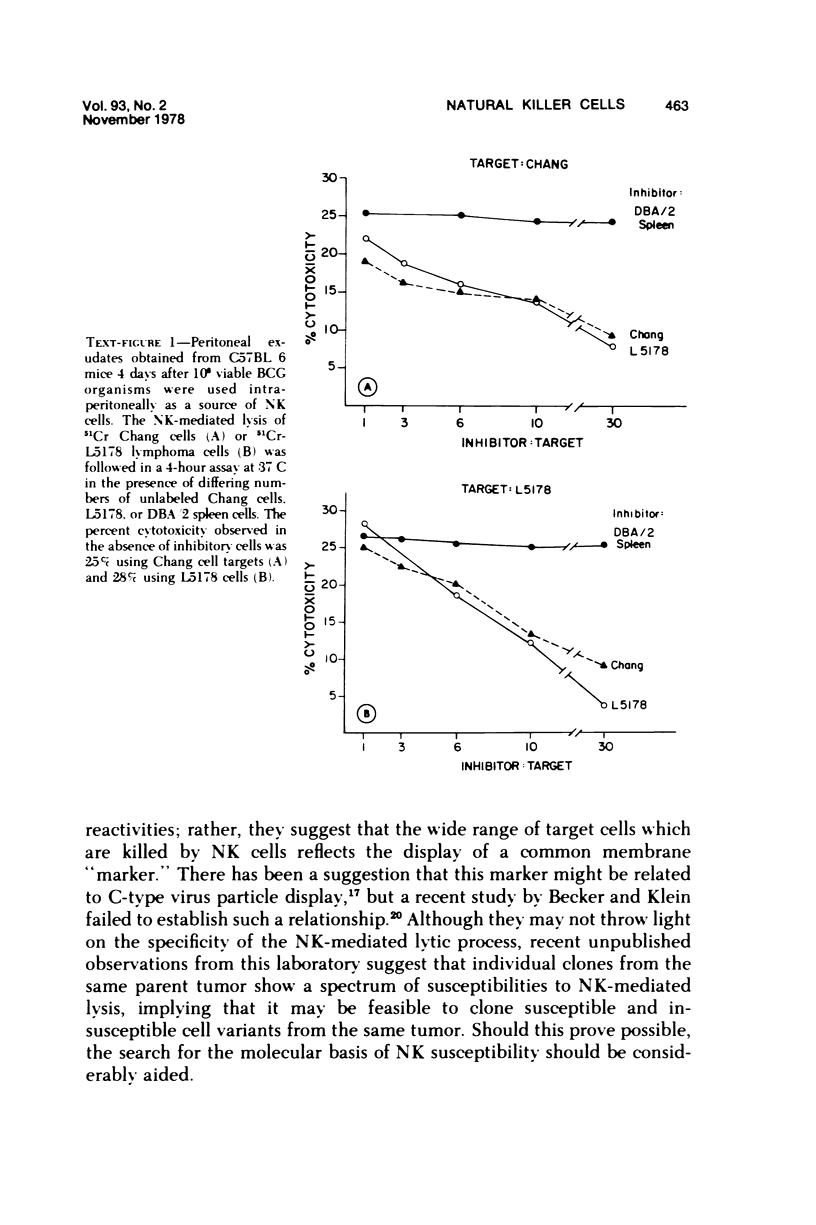
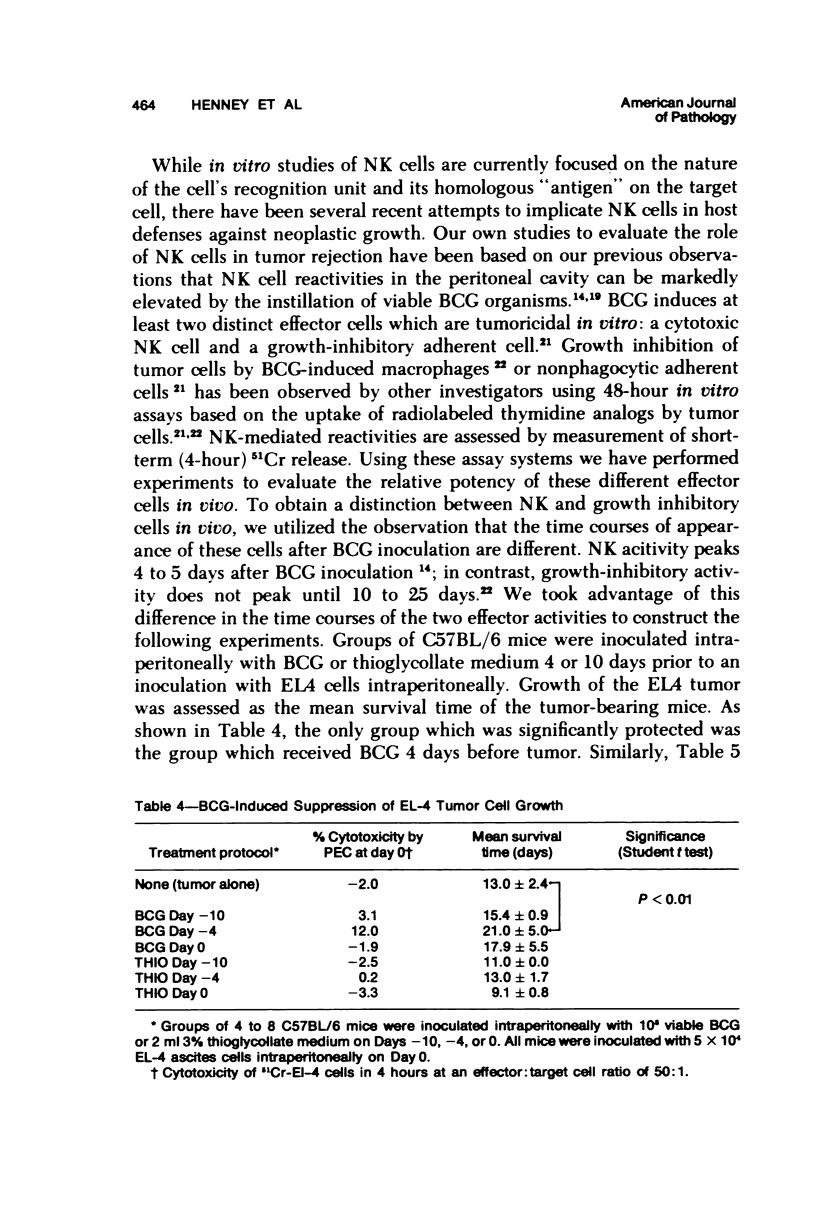
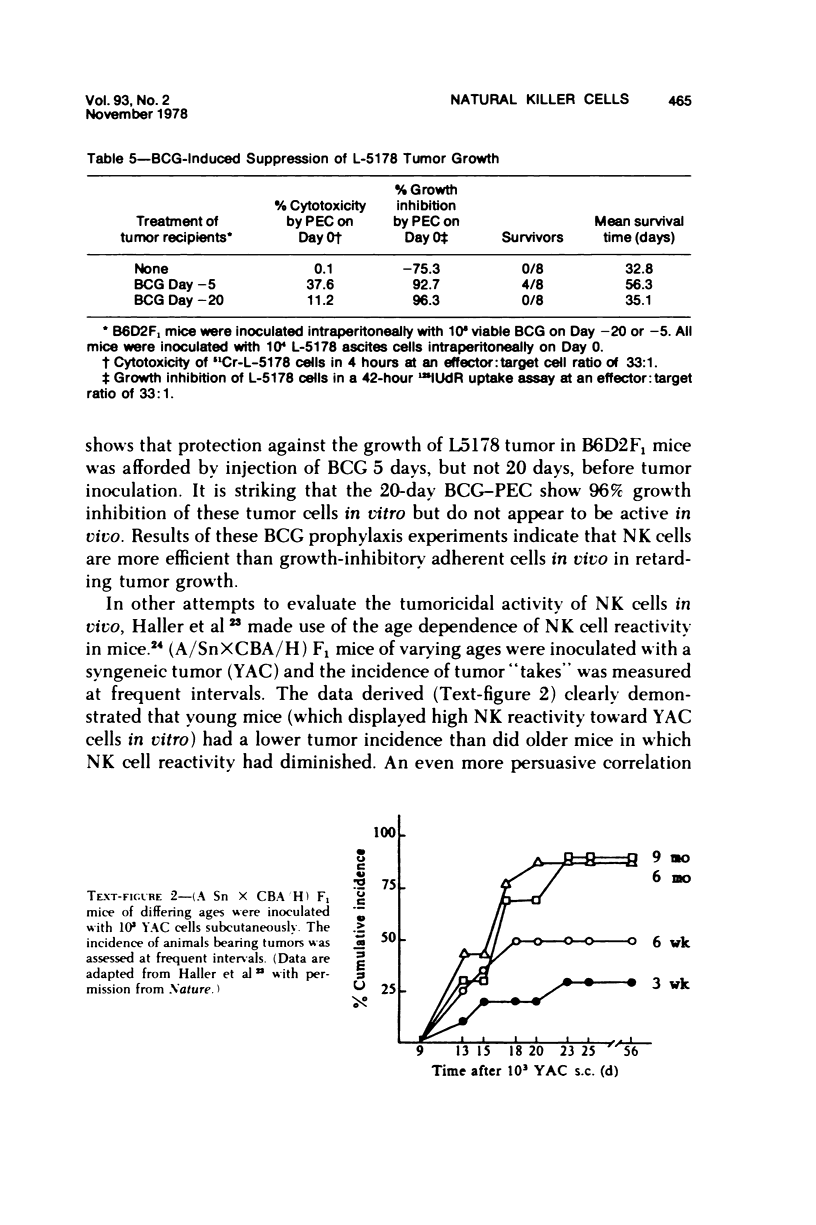
A nonadherent, nonphagocytic mouse cell found in lymphoid organelles, but lacking characteristic surface markers of mature lymphocytes, is capable of lysing a wide spectrum of tumor cells but shows little cytolytic activity toward normal cells. This cytotoxic cell, termed a "natural killer" (NK) cell, shows a marked capacity to lyse lymphomas (syngeneic, allogeneic, or even xenogeneic) to the effector cell source. Its activity is inhibited by a variety of pharmacologic agents, eg, cytochalasins, cAMP-"active" drugs, and colchicine, over the same dose range at which these drugs inhibit other cytotoxic cells. We have no evidence that NK cell "specificities" are clonally distributed. Two sets of evidence are presented which suggest that the same NK cell population is responsible for lysing a variety of tumor target cells. Preliminary evidence suggests that modulation of NK cell levels in vivo is correlated with resistance to challenge with a syngeneic tumor, inferring that NK cells may play a salient role in host defenses against neoplasia.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnet F. M. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. Specific in vitro cytotoxicity of thymus-derived lymphocytes sensitized to alloantigens. Nature. 1970 Dec 26;228(5278):1308–1309. doi: 10.1038/2281308a0. [DOI] [PubMed] [Google Scholar]
- GOVAERTS A. Cellular antibodies in kidney homotransplantation. J Immunol. 1960 Nov;85:516–522. [PubMed] [Google Scholar]
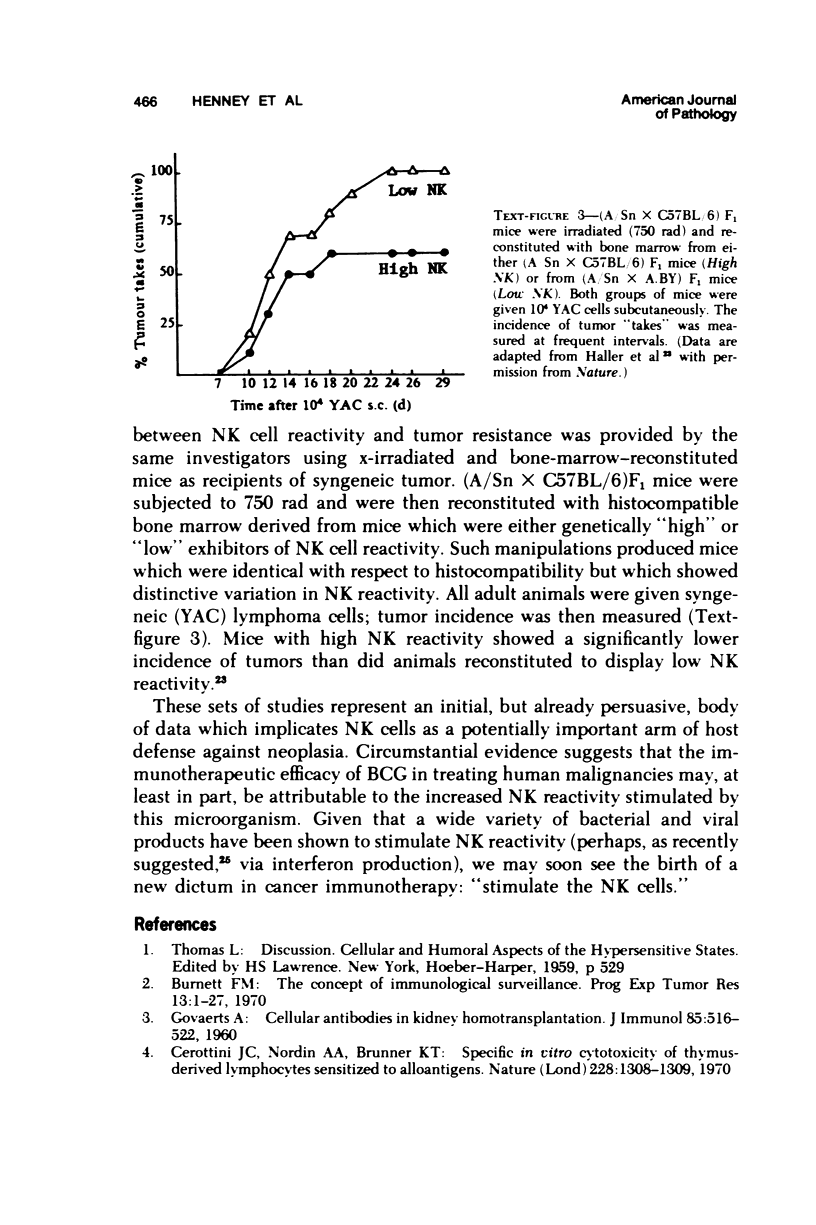
- Haller O., Hansson M., Kiessling R., Wigzell H. Role of non-conventional natural killer cells in resistance against syngeneic tumour cells in vivo. Nature. 1977 Dec 15;270(5638):609–611. doi: 10.1038/270609a0. [DOI] [PubMed] [Google Scholar]
- Haller O., Wigzell H. Suppression of natural killer cell activity with radioactive strontium: effector cells are marrow dependent. J Immunol. 1977 Apr;118(4):1503–1506. [PubMed] [Google Scholar]
- Henney C. S. Quantitation of the cell-mediated immune response. I. The number of cytolytically active mouse lymphoid cells induced by immunization with allogeneic mastocytoma cells. J Immunol. 1971 Dec;107(6):1558–1566. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975 Aug 15;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Possible role of macrophage mediated nonspecific cytotoxicity in tumour resistance. Nat New Biol. 1972 Jan 12;235(54):48–50. doi: 10.1038/newbio235048a0. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hill V. M., Terry W. D. Isolation of a subpopulation of adherent peritoneal cells with anti-tumour activity. Nature. 1976 Mar 11;260(5547):146–148. doi: 10.1038/260146a0. [DOI] [PubMed] [Google Scholar]
- Prehn R. T. The relationship of immunology to carcinogenesis. Ann N Y Acad Sci. 1969 Oct 14;164(2):449–457. doi: 10.1111/j.1749-6632.1969.tb14059.x. [DOI] [PubMed] [Google Scholar]
- Stutman O. Immunodepression and malignancy. Adv Cancer Res. 1975;22:261–422. doi: 10.1016/s0065-230x(08)60179-7. [DOI] [PubMed] [Google Scholar]
- Stutman O. Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. Science. 1974 Feb 8;183(4124):534–536. doi: 10.1126/science.183.4124.534. [DOI] [PubMed] [Google Scholar]
- Tracey D. E., Wolfe S. A., Durdik J. M., Henney C. S. BCG-induced murine effector cells. I. Cytolytic activity in peritoneal exudates: an early response to BCG. J Immunol. 1977 Sep;119(3):1145–1151. [PubMed] [Google Scholar]
- Wolfe S. A., Tracey D. E., Henney C. S. BCG-induced murine effector cells. II. Characterization of natural killer cells in peritoneal exudates. J Immunol. 1977 Sep;119(3):1152–1158. [PubMed] [Google Scholar]
- Ziegler H. K., Geyer C., Henney C. S. Studies on the cytolytic activity of human lymphocytes. III. An analysis of requirements for K cell-target interaction. J Immunol. 1977 Nov;119(5):1821–1829. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]



